Abstract
Objectives
To establish whether chondrocyte viability, matrix degradation and the induction of proteolytic gene expression in canine cartilage is independent of irrigation fluid osmolality and time following exposure to the irrigation fluid.
Methods
Canine cartilage explants were exposed to one of three different solution types i) Culture medium (270–280 mOsmol/kg) ii) NaCl 0.9% (302 mOsmol/kg) iii) NaCl 0.9% with sucrose (600 mOsmol/kg). Chondrocyte viability and selected proteolytic gene expression were measured at two time points; immediately following exposure and 24 h following exposure. The media samples at 24 h following exposure were assessed for sulphated glycosaminoglycan (sGAG) release.
Results
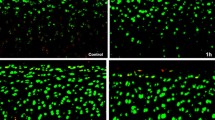
In all samples, no cell death was observed across the superficial or deeper layers of the cartilage. When adjusting for time, gene expression was not shown to be dependent on solution type. However for all solution types, Matrix Metalloproteinase 13 (MMP13) and A Disintegrin and Metalloproteinase with Thrombospondin Motifs 5 (ADAMTS5) expression was significantly decreased in cartilage samples at 24 h post exposure comparatively to samples tested immediately post exposure. No significant differences were identified in the relative sGAG release between the solution types.
Clinical Signifcance
Arthroscopic solution irrigation of cartilage explants had no effect on cell viability or proteinase production. At present there is no indication to optimise irrigation fluid osmolarity, as conventional arthroscopic solution was not deleterious to healthy cartilage in this model.





Similar content being viewed by others
References
Amin AK, Huntley JS, Bush PG, Simpson AH, Hall AC (2008) Osmolarity influences chondrocyte death in wounded articular cartilage. J Bone Joint Surg Am 90:1531–1542
Amin AK, Huntley JS, Hall AC (2010) Increasing the osmolarity of joint irrigation solutions may avoid injury to cartilage: a pilot study. Clin Orthop Relat Res 468:875–884
Amin AK, Huntley JS, Patton JT, Brenkel IJ, Simpson AHRW, Hall AC (2011) Hyperosmolarity protects chondrocytes from mechanical injury in human articular cartilage. An experimental report. The Journal of Bone and Joint Surgery [British Volume] 93:277–284
Baumgarten M, Bloebaum RD, Ross SD, Campbell P, Sarmiento A (1985) Normal human synovial fluid: osmolality and exercise-induced changes. J Bone Joint Surg Am 67(9):1336–1339
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57(1):289–300
Bulstra SK, Kuijer R, Eerdmans P et al (1994) The effect in vitro of irrigating solutions on intact rat articular cartilage. J Bone Joint Surg (Br) 76:468
Bush PG, Hodkinson PD, Hamilton GL, Hall AC (2005) Viability and volume of in situ bovine articular chondrocytes-changes following a single impact and effects of medium osmolarity. Osteoarthritis and cartilage. 13(1):54–65
Capito NM, Smith MJ, Stoker AM, Werner N, Cook JL (2015) Hyperosmolar irrigation compared with a standard solution in a canine shoulder arthroscopy model. J Shoulder Elb Surg 24(8):1243–1248
Clements DN, Fitzpatrick N, Carter SD, Day PJR (2009) Cartilage gene expression correlates with radiographic severity of canine elbow osteoarthritis. Vet J 179:211–218
Eltawil NM, Howie SEM, Simpson AHRW, Amin AK, Hall AC (2015) The use of hyperosmotic saline for chondroprotection: implications for orthopaedic surgery and cartilage repair. Osteoarthr Cartil 23:469–477
Farr J, Mathew LM, Stoker AM, Sherman SL, Cook JL (2015) Effects on exposed articular cartilage during open surgical procedures: a comparison of various fluids in an animal model. Arthroscopy 31(1):113–117
Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Hak-Ling M, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA (2005) Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434:644
Gradinger R, Träger J, Klauser RJ (1995) Influence of various irrigation fluids on articular cartilage. Arthroscopy 11(3):263–269
Gulihar A, Bryson DJ, Taylor GJ (2013) Effects of different irrigation fluids on human articular cartilage: An in vitro study. Arthroscopy 29(2):251–256
Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM (2004) Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum 50:131–141
Maccoux LJ, Clements DN, Salway F et al (2007) Identification of new reference genes for the normalisation of canine osteoarthritic joint tissue transcripts from microarray data. BMC Molecular Biology 8:62
Mitchell N, Shepard N (1989) The deleterious effects of drying on articular cartilage. J Bone Joint Surg Am 71:89–95
Shanfield S, Campbell P, Baumgarten M, Bloebaum R, Sarmiento A (1988) Synovial fluid osmolality in osteoarthritis and rheumatoid arthritis. Clin Orthop Relat Res 235:289–295
Stoker AM, Cook JL, Kuroki K, Fox DB (2006) Site-specific analysis of gene expression in early osteoarthritis using the pond-Nuki model in dogs. J Orthop Surg Res 1:8
Urban JPG, Hall AC, Gehl KA (1993) Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol 154:262–270
Acknowledgements
The Roslin Institute is supported by an Institute Core Strategic Grant from the BBSRC.
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study, collected and analysed the data and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
“All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.”
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
Figure detailing the sample collection from each stifle joint. (PNG 13 kb)
Rights and permissions
About this article
Cite this article
Henderson, E., Clements, D.N. & Johnson, C.I. An in vitro study of the effect of the optimal irrigation solution conditions during canine articular surgery. Vet Res Commun 44, 1–7 (2020). https://doi.org/10.1007/s11259-019-09765-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-019-09765-9