Abstract
In this study, we investigated the dynamics of Mycoplasma hyopneumoniae infections in 66 pig farms, with different production systems (one-, two-, and three-site systems), and considered different risk factors. Serological assay was used to detect serum antibodies against M. hyopneumoniae and real time polymerase chain reaction (RT–PCR) was performed to detect M. hyopneumoniae DNA in tracheobronchial swabs. Results demonstrated that M. hyopneumoniae infection status was predominantly influenced by the age of the animals and the type of production system. Infection rates were higher in older animals and the prevalence was higher in the one- and two-site systems than in the three-site systems. Dynamics of infection by RT-PCR showed that earlier M. hyopneumoniae infection on one-site farms occurs earlier, while on two- and three-site farms occurs later but spreads faster, suggesting that contact between animals of different age favors the transmission.
Similar content being viewed by others
Introduction
Mycoplasma hyopneumoniae is the primary etiological pathogen of enzootic pneumonia, a chronic disease of pigs characterized by high morbidity, low mortality, and a nonproductive cough (Morris et al. 1995). Mycoplasma hyopneumoniae is also considered one of the primary agents involved in the porcine respiratory disease complex (Sibila et al. 2009).
Enzootic pneumonia causes substantial economic losses, which is primarily attributable to a decreased feed conversion, and consequently a reduced weight gain. Lesions extending to 10 % of the lungs result in 37.4 g lower daily body weight gain as compared to pigs having lungs free of pathological changes (Straw et al. 1989).
Risk factors that influence the transmission of M. hyopneumoniae can be reduced through good biosecurity measures by management practices, including all-in/all-out (AI/AO), quarantining animals coming in from other farms, housing conditions, breeding practices, sanitation (e.g., disinfection of trucks, rodent and pest control and only using materials from the farm) (Giacomini et al. 2011), and proper vaccination against M. hyopneumoniae infection. Poor nursery management and no compartmentalization also contribute to the circulation, spread, and incubation of M. hyopneumoniae in herds (Nathues et al. 2013). In recent years, pig farms in Italy have undergone significant changes, with the introduction of multisite systems that incorporate AI/AO for breeding (site).
The study is performed in 3 main production systems. Single, two and three-site. Three-site system is composed of farrowing farm, nursery and finishing sites. At weaning age (3 or 4 weeks), piglets are moved to nursery site until 12 to 16 weeks and are raised at third site until slaughter age (36 weeks). In the two-site system, pigs are raised on two different farms; they live on the first farm until they are 12–16 weeks old and are then moved to the finishing farm until slaughtering. In the single-site “farrow-to-finish” system, pigs are bred and raised at the same site until slaughtering.
The structural differences in these farm systems imply that different management practices are used, potentially exposing the animals to different risk factors for M. hyopneumoniae infection. Serological response occurse in pigs 15–38 days post challenge and depend on the commercial ELISA used (Neto et al. 2014).
The gold standard for M. hyopneumoniae is isolation and bacteriological culture, but to overcome the difficulties encountered in achieving microbial growth, PCR was used to develop a faster and more sensitive test (Thacker 2004). However, the method of sampling specimens for the detection of M. hyopneumoniae with PCR is very important for accurate results. The most sensitive results are obtained using tracheobronchial washing and tracheobronchial swabs (TBS) (Marois et al. 2007). In fact, TBS may be 3.5–4.5 times more sensitive in detecting M. hyopneumoniae than nasal swabs (Marois et al. 2007). These sampling methods, together with very sensitive techniques such as RT–PCR and serology, can be used to identify the beginning of an infection and its variability according to the age of the animal. Consequently, they can be used to investigate the dynamics of infection, allowing the most appropriate control measures for M. hyopneumoniae to be devised.
The aim of this study was to investigate the dynamics of M. hyopneumoniae infection using TBS and serological testing and then if farming conditions can modify that dynamic. We found that M. hyopneumoniae infection is less prevalent in three-site system compared to one- or two-sites systems, due to the strict segregation of different animal categories.
Material and methods
Study farms and study design
In order to select homogeneous clinical conditions we chose to enroll only farms in which animal had not shown respiratory diseases in the last 6 months. The farms were selected randomly from the regional epidemiological registry in order to be representative of the Italian production systems in regards to their infection status and associated risk factors. In total, 66 farms were included in the study. According to the different distribution of farming systems in the territory, we selected 10 three-site systems, 10 two-site systems, and 46 single-site systems (Table 1). None of the selected herds displayed clinical respiratory disease in the nursery or fattening stages during our sampling time.
On every farm, pigs were assigned to 10 groups, with different age from 1 week (group 1), 1 month (group 2) to 9 months of age (groups 3–10). All the pigs were sampled during a single visit between 2010 and 2011, according to a cross-sectional design. In each group, 10 individuals were randomly selected for blood sampling and five were selected for TBS sampling, so that 100 blood samples and 50 TBS samples were collected from each farm. We determined the sample size considering 500 animals as the mean number of animals for each category. For serology we estimated a mean distribution of 60 % and with an error margin of 25 % and a confidence level of 90 %. For direct microbiological investigation (TBS) we estimated a mean distribution of 10 % with an error margin of 25 % and a confidence level of 90 %.
During the visit, a questionnaire about the health status, management, and biosecurity of the farm was completed by the same researcher (Table 1). The information on the questionnaire was verified in each unit during the herd visit. The factors examined based on the questionnaires regarding the sow units were: presence of quarantine, vaccination of gilts or boars against porcine circovirus type 2 (PCV2), mean number of sows, and the treatment and vaccination of sows against M. hyopneumoniae. The following information was collected from the nursery units: the number of animals in the nursery pens, vaccination against PCV2 and porcine reproductive and respiratory syndrome virus (PRRSV), and treatment and vaccination of piglets against M. hyopneumoniae. In the weaning unit (age < 3 months), details were collected on: age (days) at weaning, AI/AO, and the treatment and vaccination of the pigs against M. hyopneumoniae. Information from the fattening unit was divided into two parts: one group for animals weighing up to 110 kg, and another for animals weighing 111–170 kg. In both groups, we considered mean number of fatteners, treatment and vaccination against M. hyopneumoniae (Nathues et al. 2013), and months to slaughter as risk factors for infection.
Sampling procedures
For blood and TBS sampling, the animals were restrained by placing a conventional cable snare over the maxilla. The blood samples were obtained by jugular vein puncture. The blood samples were collected in sterile tubes (Vacutest Kima clot activator) and transported to the laboratory (Istituto Zooprofilattico Sperimentale Lombardia Emilia-Romagna, Brescia, Italy) at 4 °C, where they were analyzed immediately.
TBS were collected with sterile catheters used for post-cervical artificial insemination of companion animals (Sanifarm). The catheters were extracted, their ends were cut and the content placed into a tube (Vacutest Kima) containing carrier liquid (saline). They were transported to the laboratory at 4 °C where they were analyzed immediately. The samples were analyzed in the same structure (IZSLER, Brescia, Italy), in serology and biology laboratories.
Diagnostic tools
Serology
The sera were analyzed with the HerdChek® Mycoplasma hyopneumoniae Antibody Test kit (IDEXX Laboratories Switzerland AG, Stationsstrasse 12 CH-3097 Liebefeld-Bern). According to the manufacturer, sera with sample/positive control (S/P) ratios of <0.30 were considered negative within the limits of the test; samples with S/P ratios ≥0.30 and ≤0.40 were classified as suspect; and sera with S/P ratios >0.40 were considered positive (IDEXX Laboratories). The IDEXX ELISA show positive results from 30 days post infectiononwards (Neto et al. 2014).
RT–PCR from TBS
DNA was obtained from 150 μl of tracheal fluid using guanidium thiocyanate–phenol–chloroform extraction, as described by Pitcher et al. (1989). All DNA samples were analyzed for M. hyopneumoniae with RT–PCR based on the probe and primers using the CT value of 37.3 as described by Marois et al. (2010). Moreover one replicate per sample was used and the positive control was Mycoplasma hyopneumonie ATCC 25934. The technique applied was qualitative.
Statistical analysis
The PCR and serological results were analyzed statistically to compare the infection status and the antibody responses of individuals in the herds. These analyses were primarily intended to identify the relevant risk factors and then analyze the dynamics of infection within homogeneous risk groups.
Risk factors
A mixed-effects logistic model was used to identify the risk factors that were significantly associated with M. hyopneumoniae infection status, considered as binary response variables. The explanatory variables examined were vaccination against M. hyopneumoniae infection, age (as a continuous variable), farm type, compartmentalization (i.e. the number of different structure for each age group), all-in/all-out sectors, and the number of animals per farm. The farm code was considered a random intercept to overcome the possible autocorrelation and non-independence of data collected from the same herd. A likelihood ratio test was used to select the final minimal model that best explained the M. hyopneumoniae infection in the pigs (Mood 1963) as determined with PCR and serology (Calsamiglia et al. 1999).
Dynamics of infection
To examine the temporal dynamics of infection, we analyzed the change in infection comparing the ELISA status between age classes. M. hyopneumoniae infection in all herds was confirmed by RT–PCR from TBS. In order to specifically analyze the dynamics of infection among the farm types, nonparametric analysis of variance (Kruskal–Wallis) was first used to test for differences among the herds from the three-site-system farms, two-site-system farms, and one-site-system farms at the first and the last (36th) week of age. In order to test that piglets from the three farm systems had similar infection status, we compared the ELISA results in the fourth week of life to exclude contamination with maternal antibodies, which can be present up until then (Wilson et al. 2013).
A nonparametric complete block design (Friedman test) was used to separately evaluate the differences among pigs of different ages (weeks) in each type of herd. To identify difference among all age groups, multiple comparison procedure through Bonferroni-Holm was initially performed. Since these results did not differ respect Fisher’s least significant difference (LSD), we decided to report this later method in order to get simpler interpretable results. For the Kruskal–Wallis and Friedman tests, the dichotomous response variables represented by the infection status of the individual animals were transformed to the number of positive animals (CT value >37.3) for each week of age in each herd.
All statistical analyses were performed with the R software (version 3.0.0), using the functions glmer for the logistic multilevel mixed model, Kruskal test, and Friedman, to perform the Kruskal–Wallis test, Friedman test, and Fisher’s LSD, respectively. These functions are included in the lme4, stats, and agricolae packages of R, respectively. Statistical significance level was set at α = 0.05.
Results
Dynamics of infection
Of the 10 three-site-system herds, none was treated with antimicrobial medication that specifically targeted M. hyopneumoniae infection. Of those, 7 herds with suckling piglets were vaccinated against M. hyopneumoniae in the nursery stage, and the remaining three had not been vaccinated in the last 24 months. Out the 10 two-site-system herds, 7 herds were administered antimicrobial medications specifically against M. hyopneumoniae and all herds were vaccinated against M. hyopneumoniae. Out the 46 one-site-system herds, 6 herds were not vaccinated against M. hyopneumoniae or administered antimicrobial medication in the last 24 months, 2 were not vaccinated against M. hyopneumoniae but were administered medication, and 16 were both vaccinated and administered specific medications. Of the 22 herds that were vaccinated, the pigs in 20 of them were vaccinated before they were 30 days old. The medication used by the farms consisted of drugs belonging exclusively to the macrolide, lincosamide, and sulfonamide groups. Table 2 lists the reported antimicrobial treatments and vaccination programs against M. hyopneumoniae in three categories, based on the pig ages: less than 28 days of age, from 29 to 90 days of age, and more than 90 days of age.
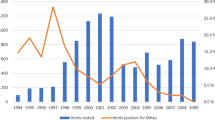
The dynamics of infection was explored through a Fisher’s LSD pairwise comparison between the age groups, which showed that seroprevalence in the three-site system did not change between four and 20 weeks of age, but increased between weeks 20 and 24. After week 24, the number of seropositive animals did not change until week 32, when it decreased until week 36 (Figs. 1 and 2). In the one-site-system herds, Fisher’s LSD comparisons indicated that there was a constant increase in the rate of seropositivity with age until week 24, after which the number of seropositive pigs did not change significantly (Fig. 2). In the two-site-system herds, post hoc comparisons showed that the seroprevalence observed in four-week-old pigs did not change until week 16, when it increased continuously until week 24, after which the number of seropositive pigs did not increase significantly.
According to the ELISA results, in the first week of life, there was no statistically significant difference among the three herd types (p > 0.05; Fig. 2). In the 36th week, farm systems showed significant differences (p = 0.02), with two-site-system farms having higher rates of seropositivity while three-site-system farms had the lowest rates. In the herds of all systems, the Friedman test showed a significant change in the number of seropositive pigs with age (p < 0.001), and this difference was apparent approximately 4 weeks later than the same change that was detected with PCR.
The number of PCR-positive pigs changed with the age (weeks) in the herds in all three systems (for all, p < 0.001). However, in the first and last weeks of age, there were no significant differences in the PCR infection status of the three farm types (p = 0.19 and p = 0.47 respectively), but the dynamics of infection differed. In the three-site-system herds, Fisher’s LSD pairwise comparisons showed that the number of PCR-positive pigs did not change from 1 week until 16 weeks of age. A change was detected at the 20th week, but the number of infected pigs did not change thereafter (Fig. 1). In the two-site-system herds, Fisher’s LSD pairwise comparisons indicated that the number of PCR-positive pigs did not change in the first 12 weeks of age. However, at week 12, it markedly increased, but remained stable thereafter (Fig. 1). In the one-site-system herds, Fisher’s LSD pairwise comparison showed a continuously increasing trend, in which the number of PCR-positive animals did not stabilize at any specific age.
Risk factors
The final minimal model describing ELISA infection status included age and farming system (Tables 3 and 4) as risk factors, while the presence of quarantine, vaccination of gilts or boars against porcine circovirus type 2 (PCV2), mean number of sows, treatment and vaccination of sows against M. hyopneumoniae were excluded.
The multilevel logistic model showed that the three types of farms differed, and that animal age exerted a significant effect on the probability of being infected by M. hyopneumoniae (Table 4). In particular, an odds ratio for age of 1.119 (95 % confidence interval [CI] = 1.113–1.127; p < 0.001) indicated an increasing prevalence of seropositivity with age. An odds ratio of 3.402 (95 % CI = 1.562–7.412) showed that infection was more common in herds on one-site-system farms than in herds on three-site-system farms (p = 0.002), although there was no statistical difference between the herds on one-site-system farms and those on two-site-system farms (p = 0.93). An odds ratio of 3.522 (95 % CI = 1.306–9.465) indicated that infection was more common in herds from two-site-system farms than in herds from three-site system farms (p = 0.02).
The final minimal model (Table 3) describing M. hyopneumoniae infection status only included age and the type of farm, while vaccine use, compartmentalization, AI/AO sectors, and the number of animals per farm were excluded. The odds ratios for age was 1.065 (95 % CI = 1.0598–1.0773), indicating proportionally higher infection rates in older animals than in younger animals (p < 0.0001). The odds ratios for farm type were 2.22 (95 % CI = 1.296–3.854) and 1.97 (95 % CI = 1.013–3.832) for the one-site system and two-site system, respectively, indicating a higher rate of infection on these farms than on three-site-system farms. There was not a statistically significant difference between the one-site-system and two-site-system herds (p = 0.63).
Discussion
This study shows that the farming system is the major factor influencing infection and seropositivity for M. hyopneumoniae infection on pig farms. The age of the pigs also influences the probability of infection and seropositivity, and both increase with age. Although all the farm systems had similar infection rates in piglets and in those at slaughter age, the temporal dynamics of infection differed strongly among the three farming systems. In this study, we investigated the infection dynamics separately in three age groups: in animals less than 4 week of age, in animals between 4 and 16 weeks, and in animals older than 16 weeks. Monitoring the immune status of a herd with regard to M. hyopneumoniae infection is an important first step in optimizing control measures, such as vaccination and medication. The enzyme-linked immunosorbent assay (ELISA) is the serological test most commonly used to detect anti-M. hyopneumoniae antibodies (Sørensen et al. 1997) .
The risk factors that were analyzed, including vaccination against M. hyopneumoniae and AI/AO, showed no evidence of influencing the nursery and weaning compartments. In particular, M. hyopneumoniae vaccination showed no significant relationship with the dynamics of infection, and its application did not reduce the infection rate in two-site farms where 90 % of herds sampled were vaccinated. It has been reported that in most herds, vaccination reduces performance losses attributable to M. hyopneumoniae and may vary from herd to herd (Maes et al. 2008). However, Villareal et al. (2011) and Pieters et al. (2010, 2014) showed that vaccination had no or only limited effects on the transmission of this organism. To acquire all the information regarding the sanitary status and the sanitary management of the farms were the investigation was carried out, we administred a questionnaire.
In particular, we acquired information regarding the use of vaccination and antimicrobial treatment. We found that vaccination was performed similarly among the three farming systems therefore we can infer that it could barely interfere with our results.
On the contrary antimicrobial treatments were differently given to the three farming systems being often performed in the two-site systems (70 %), rarely performed in the one-site systems and never performed in the three-site systems. This could imply that such treatments can interfere with our results. By the way, it should be cleared that the information acquired were related to past management practices and therefore they should not interfere our data acquired from the administration of the questionnaire onward.
The lower infection rates on the farms that weaned the piglets at 3 weeks of age compared with the farms that weaned at 4 weeks of age indicates that, the age of weaning influences the probability of future infection of the animals. Nathues et al. 2013 demostrated the nasal swabs positivity of piglets increased by 10 % for every day the suckling period was lasting longer (Nathues et al. 2013). In 2015 Vangroenweghe et al. has been shown that under US conditions low infection rate is present when piglets are weaned before 21 days of age (Vangroenweghe et al. 2015). The beneficial effect of AI/AO, practiced in herds or in fattening units to limit the spread of the disease in general, is well known (Grosse Beilage et al. 2009, Clark et al. 1991). Our data support this information also for M. hyopneumoniae infection.
The number of animals and the size of the farm did not influence the infection dynamics and are often unrelated to good practice in animal management and AI/AO on farms. No statistical relationships were observed between the infection rate and the number of animals on the farms including the numbers of fattening pigs produced or the animal densities in the pens. This is contrary to the report of Maes et al. (2000), who cited pig density as a risk factor for M. hyopneumoniae infection.
The dynamics of M. hyopneumoniae infection are not only related to AI/AO, but can also be affected by vaccination (Nathues et al. 2013). Conversely, our results show that the farming type exerted the predominant effect on M. hyopneumoniae infections, with negligible involvement of vaccination. In the one-site farms, the infection of piglets tended to be higher and the prevalence increased progressively with advancing age (Maes 2010). In the three-site systems, infection in the nursery and growing pigs was less prevalent than in single-site herds. However, once the pigs were moved to fattening units, the prevalence increased abruptly, as previously observed (Sibila et al. 2004), without reaching the values observed in three-site systems. This suggests that in the three-site system, this factor is so influential that it overshadows all other risk factors, making the farming system the only influential factor. However, it is also likely that other management practices do not differ quantitatively among the different farming types, so these factors do not exert different effects.
The effect of age on infection is particularly important because young animals are the most vulnerable individuals, and acquire M. hyopneumoniae when exposed to the oldest and heavily colonized individuals (Meyns et al. 2004; Fano et al. 2007; Sibila et al. 2007; Nathues et al. 2013). Therefore, because the farming type influences the exposure of susceptible pigs to older and infected individuals, it is a major risk factor in the spread of M. hyopneumoniae. This effect was mainly evident in the dynamics of infection, with early infections in the one-site system and later infections in the two- and three-site systems. Thus, the spread of M. hyopneumoniae evolves slowly on two-site-system farms, as is commonly recognized (Wallagren et al. 1993). In contrast, in the two- and three-site systems, infection spreads within a single time interval (4 weeks), in which its prevalence increases from 0 % to 30 %, with an unexpectedly fast pattern of spread when the animals were moved to other sites. Although the infection is delayed in the two- and three-site systems, this accelerated pattern of spread compensates for the delay not seen in the one-site system, resulting in similar infection rates at slaughter age, regardless of the farm type. Because the infection by M. hyopneumoniae on one-site system farms is protracted, it could be argued whether this influences the onset and degree of lung lesions (Wilson et al. 2012).
Serology showed an higher prevalence of M. hyopneumoniae than that observed using PCR. This obvious finding provide however justification for the use of both diagnostic approachwhen considering risk factors influencing the dynamic of M. hyopneumoniae infections.
Conclusions
This study demonstrates that the production systems used in the pig industry influences the M. hyopneumoniae infection. The farming system mainly affects the dynamics of infection, with earlier infection in one-site systems and later infection in two- and three-site systems. This is particularly important in Italy, when the slaughter age is different from other European countries, where many pigs are slaughtered at 9 months of age. These patterns were identified with PCR using tracheobronchial swabs combined with serological analyses. The consistent results obtained in this study between the two diagnostic techniques support the use of serological testing combined with PCR of TBS as a simple monitoring tool. Because different farm systems demonstrated a similar prevalence of M. hyopneumoniae at slaughter age, further research is required to determine whether these different infection dynamics cause different pathological patterns in pig tissues at the abattoir. These data should contribute to provide essential guidelines on the best farming system to reduce the sanitary impact of M. hyopneumoniae infections in pig farms.
References
Calsamiglia M, Pijoan C, Trigo A (1999) Application of a nested polymerase chain reaction assay to detect Mycoplasma hyopneumoniae from nasal swabs. J Vet Diagn Investig 11:246–251
Clark L, Freeman M, Scheidt A, Knox K (1991) Investigating the transmission of Mycoplasma hyopneumoniae in a swine herd with enzootic pneumonia. Vet Med 86:543–550
Fano E, Pijoan C, Dee S, Deen J (2007) Effect of Mycoplasma hyopneumoniae colonization at weaning on disease severity in growing pigs. Can J Vet Res 55:195–200
Giacomini E, Ferro P, Nassuato C, Salogni C, Alborali L (2011). Dynamics of Mycoplasma hyopneumoniae infection in 4 Italian swine farrow-to-finish herds. In Proceedings of the Società Italiana di Patologia ed Allevamento dei Suini: 25–25 March 2011; Piacenza. Edited by SIPAS. Piacenza 286–298
Grosse Beilage E, Rohde N, Krieter J (2009) Seroprevalence and risk factors associated with seropositivity in sows from 67 herds in north-west Germany infected with Mycoplasma hyopneumoniae. Prev Vet Med 88:255–263
Maes D (2010) Mycoplasma hyopneumoniae in pigs 2010. Update on epidemiology and control. In Proceedings of the International Pig Veterinary Society Congress: 18–21 July 2010, Vancouver. Edited by D’Allaire S. Friendship R. Vancouver 30–35
Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Vrijens B, de Kruif A (2000) Herd factors associated with the seroprevalences of four major respiratory pathogens in slaughter pigs from farrow-to-finish herds. Vet Res 31:313–327
Maes D, Segalés J, Meyns T, Sibila M, Pieters M, Hasebrouck F (2008) Control of Mycoplasma hyopneumoniae infection in pigs. Vet Microbiol 126:297–309
Marois C, Le Carrou J, Kobisch M, Gautier-Bouchardon AV (2007) Isolation of M. hyopneumoniae from different sampling sites in experimentally infected and contact SPF piglets. Vet Microbiol 120:96–104
Marois C, Dory D, Fablet C, Madec F, Kobisch M (2010) Development of a quantitative real-time TaqMan PCR assay for determination of the minimal dose of Mycoplasma hyopneumoniae strain 116 required toinduce pneumonia in SPF pigs. J Appl Microbiol 108(5):1523–1533
Meyns T, Maes D, Dewulf J, Vicca J, Haesebrouck F, de Kruif A (2004) Quantification of the spread of Mycoplasma hyopneumoniae in nursery pigs using transmission experiments. Prev Vet Med 55:265–275. doi:10.1016/j.prevetmed.2004.10.001
Mood AM (1963) Introduction to the theory of statistics, 2nd edn. McGraw-Hill, New York
Morris CR, Gardner IA, Hietala SK, Carpenter TE (1995) Enzootic pneumonia: comparison of cough and lung lesions as predictors of weight gain in swine. Can J Vet Res 593:197–204
Nathues H, Doehring S, Woeste H, Fahrion AS, Doherr MG, Grosse BE (2013) Individual risk factors for Mycoplasma hyopneumoniae infections in suckling pigs at the age of weaning. Acta Vet Scand 55:44. doi:10.1186/1751-0147-55-44
Neto G, Strait L, Raymond M, Ramirez A, Minion C (2014) Antibody responses of swine following infection with Mycoplasma hyopneumoniae, M. hyorhinis, M. hyosynoviae and M. flocculare. Vet Microbiol 174(2014):163–171
Pieters M, Fano E, Pijoan C, Dee S (2010) An experimental model to evaluate Mycoplasma hyopneumoniae transmission from asymptomatic carriers to unvaccinated and vaccinated sentinel pigs. Can J Vet Res 74:157–159
Pieters M, Cline GS, Payne BJ, Prado C, Ertl JR, Rendahl AK (2014) Intra-farm risk factors for Mycoplasma hyopneumoniae colonization at weaning age. Vet Microbiol 172(3–4):575–580. doi:10.1016/j.vetmic.2014.05.027
Pitcher DG, Saunders NA, Owen RJ (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156
Sibila M, Calsamiglia M, Vidal D, Badiella L, Aldaz A, Jensen JC (2004) Dynamics of M. hyopneumoniae infection in 12 farms with different production systems. Can J Vet Res 68:12–18
Sibila M, Nofrarias M, Lopez-Soria S, Segalés J, Riera P, Llopart D, Calsamiglia M (2007) Exploratory field study on Mycoplasma hyopneumoniae infection in suckling pigs. Vet Microbiol 55:352–356. doi:10.1016/j.vetmic.2006.12.028
Sibila M, Pieters M, Molitor T, Maes D, Haesebrouck F, Segalés J (2009) Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet J 181:221–231
Sørensen V, Ahrens P, Barfod K, Feenstra AA, Feld NC, Friis NF, Bille-Hansen V, Jensen NE, Pedersen MW (1997) Mycoplasma hyopneumoniae infection in pigs: duration of the disease and evaluation of four diagnostic assays. Vet Microbiol 54:23–34
Straw BE, Touvinen VK, Bigras-Poulin M (1989) Estimation of the cost of pneumonia in swine herds. J Am Vet Med Assoc 195:1702–1706
Thacker EL (2004) Diagnosis of Mycoplasma hyopneumoniae. J Swine Health Prod 12(5):252–254
Vangroenweghe F, Karriker L, Main R, Christianson E, Marsteller T, Hammen K, Bates J, Thomas P, Ellingson J, Harmon K, Abate S, Crawford K (2015) Assessment of litter prevalence of Mycoplasma hyopneumoniae in preweaned piglets utilizing an antemortem tracheobronchial mucus collection technique and a real-time polymerase chain reaction assay. J Vet Diagn Investig 27(n° 5):606–610
Villareal I, Meyns T, Dewulf J, Vranckx K, Calus D, Pasmans F, Haesebrouck F, Maes D (2011) The effect of vaccination on the transmission of Mycoplasma hyopneumoniae in pigs under field conditions. Vet J 188:48–52
Wallagren P, Sahlander P, Hassleback G, Heldner E (1993) Control of infection with Mycoplasma hyopneumoniae in swine herds by disrupting the chain of infection, disinfection of buildings and strategic medical treatment. ZBL J Vet Med 40:157–169
Wilson S, Van Brussel L, Saunders G, Taylor L, Zimmermann L, Heinritzi K, Ritzmann M, Banholzer E, Eddicks M (2012) Vaccination of piglets at 1 week of age with an inactivated Mycoplasma hyopneumoniae vaccine reduces lung lesions and improves average daily gain in body weight. Vaccine 30 (Suppl 52): 7625–7629
Wilson S, Van Brussel L, Saunders G, Runnels P, Taylor L, Fredrickson D, Salt J (2013) Vaccination of piglets up to 1 week of age with a single-dose Mycoplasma hyopneumoniae vaccine induces protective immunity within 2 weeks against virulent challenge in the presence of maternally derived antibodies. Clin Vaccine Immunol 20(5):720–724. doi:10.1128/CVI.00078-13
Acknowledgments
This work was supported by the diagnostic laboratory of IZSLER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of this paper have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of this paper.
Rights and permissions
About this article
Cite this article
Giacomini, E., Ferrari, N., Pitozzi, A. et al. Dynamics of Mycoplasma hyopneumoniae seroconversion and infection in pigs in the three main production systems. Vet Res Commun 40, 81–88 (2016). https://doi.org/10.1007/s11259-016-9657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-016-9657-6