Abstract
Brucella abortus, a gram negative, facultative intracellular pathogen causes brucellosis in many animal species and humans. Although live, attenuated vaccines are available against this infection, they suffer from certain limitations. Therefore, the development of an effective subunit vaccine against brucellosis is an area of intense research. The outer membrane proteins (OMPs) of Brucella species have been extensively studied for its immunogenicity and protective ability. We have investigated the potential of CpG ODN to enhance the immunogenicity and protective efficacy of recombinant 28 kDa outer membrane protein (rOMP28) of Brucella melitensis. The study demonstrated vigorous immunoglobulin G (IgG) response of OMP28. The administration of rOMP28 with CpG caused increased cell mediated immune response in terms of induced IgG2a, T-cell proliferation and up-regulation of type I cytokine expression. In contrast, the free antigen suppressed the interferon gamma (type I cytokine) production on in-vitro stimulation of spleenocytes. The result indicates the role of OMP28 in the down regulation of IFN-γ production. Moreover, the B. abortus S-19 vaccinated mice showed highest production of IL-4 and IFN-γ. The protective ability of the antigen was evaluated by systemic bacterial clearance after challenging the mouse with B. abortus 544 pathogen. The level of protection was significant in rOMP28+CpG treated mice but was lower than the required level. The results of the present study indicate that rOMP28 could be an immunogen capable of inducing both humoral and cellular immune response. The humoral response was biased towards Th1 type when it was co-administered with CpG.
Similar content being viewed by others
Introduction
Brucellae are facultative intracellular Gram negative bacterium and considered as an important cause of abortion and infertility in livestock. Brucellosis is emerging globally as a significant disease of public health and economic importance (Henk et al. 2004). Because of intracellular localization, it has been suggested that CMI response is crucial in control of infection implying thereby that Th1 response is required (Splitter et al. 1996). Owing to highly infectious nature of Brucella and lack of effective human vaccine, the organism has been categorized as a potential bio-warfare agent (Paranavitana et al. 2005).
B. abortus S-19 and RB-52 are the two WHO recommended vaccine against brucellosis in cattle (Corbel 1997a), however of limited application, as they may induce abortion when administered to pregnant animals (Corbel 1997b). Besides, interference during sero-diagnosis to identify vaccinated and infected animals, virulence for human limits the use of these vaccines (Mallick et al. 2007). As a result, an attempt to develop some alternative vaccine is on by various researchers and their working groups all over the world. Several Brucella antigens such as p39 (Al-Mariri et al. 2001), Cu-Zn SOD (Tabatabai and Pugh 1994), L7/L12 (Bachrach et al. 1994; Oliveira and Splitter1996; Oliveira et al. 1992; Oliveira et al. 1994; Oliveira and Splitter 1994; Mallick et al. 2007), GroEL & GroES (Oliveira et al. 1996), YaJC (Vemulapalli et al. 2000), OMP31 (Cassataro et al. 2005b) have been attempted to find out protein component that can be used to protect the target animal species. Efforts were also made to use these immunodominant proteins in combination with adjuvants such as MBP with rL7/L12 protein (Oliveira and Splitter 1994), OMP with monophosphoryl lipid A, trehalose dicorynomycolate & PEC microparticles (Munoz et al. 2006; Brooks-Worrell and Splitter 1996), CpG with p39 (Al-Mariri et al. 2001), liposomization of L7/L12 (Mallick et al. 2007), but only L7/L12 (Oliveira and Splitter 1996), certain epitopes of Cu- Zn SOD (Onate et al. 1999; Tabatabai and Pugh 1994; Vemulapalli et al. 2000) and OMP31 (Cassataro et al. 2005b) induced some level of protection against infection in a mouse model. Although, the type of antigen administered to a host influence the type of immune response, the presence of certain cytokines at the site of injection is crucial for orienting the emerging T cell response (Janeway et al. 1998; Wang et al. 2008). Therefore, uses of adjuvants are of paramount importance in vaccinology. It has been shown that a synthetic phosphorothioate oligodeoxynucleotide containing an unmethylated, consensus immunostimulatory CpG motif (5′-Purine- Purine- CpG- Pyrimidine- Pyrimidine-3′ oligodeoxynucleotide [CpG ODN]) induce strong adjuvant activity eliciting CMI response (Jakob et al. 1998; Krieg 1999; Krieg 2000) with a Th1 like cytokine profile (Chu et al. 1997; Kovarik et al. 1999) making CpG ODN a suitable adjuvant for intracellular pathogens (Elkins et al. 1999). CpG ODN produces strong adjuvant activity eliciting cell mediated immune response when co-administered along with antigens (Al-mariri et al. 2001). Also, the protection afforded could be improved by using a multiple subunit vaccine, it remains possible that a more powerful antigen with or without adjuvant may result in protection with monovalent subunit vaccine. B. melitensis OMP28 has been shown to induce cellular immunity to infection by smooth strain and humoral immunity to rough strain of Brucella (Towbin et al. 1979). The immunogenisity of Brucella OMP28 has been reported by Lindler et al. (1996) however, its protective propensity is still to be worked out.
In the present study, the immunogenicity and protective ability of rOMP28 with or without CpG ODN was demonstrated.
Materials and methods
Animals
Inbred female Swiss Albino mice (4–6 weeks old), were procured from the Laboratory Animal Resources (LAR), Indian Veterinary Research Institute (IVRI), India. New Zealand white rabbits were used for raising hyperimmune sera. All the animals were housed under standard conditions at the institute’s animal facility having free access to feed and water ad libitum.
Chemicals and reagents
All the reagents used in the present study were of analytical and molecular biology grade. The CpG ODN was purchased from Operon Technologies (Germany), which was phosphorothioate-modified and was dissolved in sterile PBS (0.01 M, pH 7.2). The ODN used in this study were the immunostimulatory CpG 1826 5′ TCCATGACGTTCCTGACGTT 3′ (CpG motif are underlined).
Bacterial strains and plasmid
The virulent B. abortus Strain544 and the attenuated B. abortus Strain19 were maintained in the Brucella Laboratory, Division of Veterinary Public Health, Indian Veterinary Research Institute (IVRI), Izatnagar, India. The strains were tested for purity and biochemical characters by standard biochemical tests before use in the experiment. Brucella species were grown in Brucella broth/agar medium (Difco laboratories, Detroit, M). Recombinant Escherichia coli, expressing 28 kDa OMP of B. melitensis (provided by P.Chaudhuri, IVRI, Izatnagar) were grown on LB medium supplemented with required antibiotics.
Expression and purification of rOMP28
PCR amplification and cloning
B. melitensis 16 M DNA encoding 28 kDa outer membrane protein gene was amplified by PCR, using a set of primers selected on the basis of nucleotide sequence described in the literature (Chaudhuri et al. 2005). In order to overproduce the 28 kDa outer membrane protein (OMP28) of B. melitensis, the amplified DNA was cloned into the expression vector, pPROExHTb (Life Technology, USA). Restriction sites for BamH I and for Hind III were introduced to oligonucleotides to facilitate cloning. PCR was performed for 30 cycles at 94°C for 1 min., 55°C for 1 min and 72°C for 1 min. The PCR amplified product was eluted from agarose gel and ligated to the pPROExHTb vector with T4 DNA ligase. The newly constructed plasmid was designated as pOMP28 and was transformed into E. coli DH 5α cells.
Purification of rOMP28 by Ni-NTA column chromatography
Escherichia coli DH5α cells harboring recombinant plasmid pOM28 were grown in LB medium till OD600nm reaches to 0.5. The cells were then induced with 1 mM IPTG and allowed to grow further for 6 hours at 37°C. Polyhistidine tagged recombinant OMP28 was purified under denaturation condition by Ni-NTA affinity chromatography (Qiagen, USA).
SDS-PAGE analysis of the protein
SDS-PAGE analysis was performed as described by Laemmli (1970) in a vertical mini gel electrophoresis apparatus (ATTO, Japan) using 12.5% resolving gel having a stacking gel (5%). Electrophoresis was carried out at a constant current of 20 mA and stained with 0.25% (w/v) Coomassie Brilliant Blue R-250.
Immunization
The immunological studies were performed in inbred female Swiss Albino mice. Each group consisted of twenty mice. The immunization schedules adapted for administration of rOMP28 was same for all groups of mice and described accordingly. Different groups of experimental mice were injected separately, through intramuscular route, for immunization on days 0 and 21 with CpG ODN adjuvanted rOMP28 (group 1), rOMP28 (group 2), CpG ODN alone (group 4). Mice vaccinated with B. abortus S-19 (5 × 106 CFU/mice) were included as positive control (group 5), while another set of animal (negative control) received only PBS treatment (group 3). Animals were immunized with priming dose of 30 µg of rOMP28 and 25 µg of CpG-ODN per mice and boosted subsequently on days 21 with the same dose and route.
Collection of sera
Mice were bled through retro-orbital puncture with capillary tubes and blood was collected in sterile microfuge tubes. Blood was incubated at room temperature for an hour and subsequently kept at 4°C for retraction. Finally, the serum was collected and centrifuged at 5000xg for 10 min at 4°C to remove residual blood cells. The final preparation was kept at −20°C till further use.
Raising of hyperimmune sera
Two healthy adult rabbits (New Zealand White) procured from Institutes’ LAR section were injected intramuscularly with purified rOMP28 (100 µg) for the production of anti-rOMP28 hyper-immune serum. A booster was given on day 14th. The rabbits were bled for serum collection on day 21st and serum was analyzed for presence of antigen specific antibodies by Western blot.
Determination of antigen specific antibody by Western Blot
The production of antigen specific antibodies in hyper-immune serum of the rabbit was determined by Western Blot analysis (Towbin et al. 1979). Briefly, purified and dialyzed rOMP28 was run with pre-stained marker on 12.5% SDS-PAGE and subsequently electro-blotted onto nitrocellulose membrane at 2 mA/cm2 constant current for an hour using semidry electro blot assembly (ATTO, Japan) containing transfer buffer (25 mM Tris, 192 mM glycine and 20% methanol). The membrane was blocked with 2% bovine serum albumin (Sigma, St. Louis) for 4 h at 4°C and washed five times with Phosphate Buffered Saline (PBS 0.01 M, pH 7.2) containing Tween 20 (PBS-T) and was incubated with hyper-immune (1: 200 dilution) sera for 2 h at 4°C. The membrane was washed with PBS-T and incubated with horse radish peroxidase (HRP) conjugated goat anti-rabbit IgG antibody (1:2,500 dilution) for 1 h at 4°C and was finally washed with PBS-T. Diaminobenzidine (6 mg/10 ml Tris buffer [0.01 M, pH-7.6]) in the presence of 4 µl of 30% H2O2 was used for development of membrane.
Determination of antigen specific immunoglobulin and their isotypes by indirect ELISA (iELISA)
Sera of immunized mice were collected at regular interval to monitor the antibody response by iELISA as the method described earlier (Cassataro et al. 2004). Briefly, 96-well microtitre plate (Nunc-Maxisorp, Rosklide, Denmark) was coated overnight with 100 µl of purified rOMP28 antigen (1.25 µg/ml) in carbonate-bicarbonate buffer (0.05 M, pH 9.6) at 4°C. Next day, plate was washed thrice with PBS-T and blocked with skimmed milk powder (3%) in PBS-T for 1 h at 37°C. After 3–4 washings with PBS-T, the plate was incubated with serial dilutions of test and negative control sera starting from 1/1,000 and 1/200 dilution, respectively, at 37°C for 2 h. After 3–4 washings of the plate, anti-mouse IgG (H+L), IgG1 and IgG2a HRPO conjugate (100 µl/well) were added to wells and incubated at 37°C for 1 h. After incubation, the plate was washed 3–4 times and 100 µl of freshly prepared substrate solution (6 mg OPD/10 ml substrate buffer with 4 µl of 30% H2O2) was added to each well and incubated for 15 min in the dark. The reaction was stopped by addition of 50 µl of H2SO4 (1 M) per well. The absorbance was measured using ELISA reader (Biorad) at 492 nm. The titer was expressed as optical density (OD) units, which was obtained by multiplying the reciprocal dilution of the serum by the OD (A492) at that dilution (Bhattacharjee et al. 2006).
Lymphocyte proliferation assay
The lymphocyte proliferation was estimated by colorimetric assay as described earlier (Mosmann 1983). On day 7 and 21 after booster, three mice from each group were used to perform lymphocyte proliferation assay. Spleen were removed aseptically from anaesthetized mice and washed with PBS. Spleenocytes were collected from spleen by forceful flushing of spleen with chilled PBS. The flushed PBS containing spleenocytes and RBCs was layered slowly on to equal volume of Histopaque-1077 (Sigma, USA) and centrifuged at 1000xg for 40 min. The interface containing spleenocytes were collected and washed with chilled PBS followed by a final wash with RPMI-1640 containing 10% newborn calf serum (NBCS), L-glutamine (2 mM), streptomycin (100 µg/ml) and penicillin (100 IU/ml). Viable cells were counted by 0.1% Trypan Blue dye exclusion method and count was adjusted to 2 × 105 cells/100 µl per well in 96-well flat bottom cell culture plate (Nunc, Denmark). Cells were stimulated in-vitro with rOMP28 (1 µg/ml), concanavalin-A (ConA) (2.5 µg/ml) or medium alone in triplicate and incubated at 37°C with 5% CO2. After 72 h incubation, 10 µl of MTT (Thiazolyl blue @ 5 mg/ml in RPMI 1640) was added to all the wells and the plate was further incubated at 37°C for 4 h. Thereafter, the plate was centrifuged at 1,000 × g for 10 min in refrigerated centrifuge (Eppendorf, Germany) to settle down the formazen crystals. The supernatant was aspirated and the crystals were dissolved with 150 µl/well of dimethyl sulfoxide (DMSO, 100%) by vigorous pipetting. Finally, the absorbance was measured at 570 nm with a reference wavelength of 650 nm using ELISA reader (Biorad). The stimulation indices (SI) were calculated as the ratio between the absorbance values of stimulated cells to the unstimulated cells using the following formula:
In vitro assay for IFN-γ and IL-4 production by spleen cells
Gamma-interferon (IFN-γ) and interleukin (IL-4) were estimated by antigen capture ELISA using cytokine assay kit (Cytolab, USA). The presence of these cytokines was assayed in the culture supernatants of spleen cells collected after 72 h of antigen stimulation. The cytokines were quantified in triplicate by ELISA using microplate (Nunc-Maxisorp, Rosklide, Denmark) coated with capture antibody (anti-mouse IFN-γ and anti-mouse IL-4) as per the manufacturer’s guide. The concentration of IFN-γ and IL-4 in the culture supernatants were calculated for each experimental group using a linear-regression equation obtained from the absorbance values of the standards.
Protection against challenge with B. abortus 544 infection
Reduction in log10 CFU in spleen of challenged mice of experimental group with respect to control was taken as protection (Cassataro et al. 2005a).
Three weeks after the booster, 6 mice from each group were challenged with a virulent B. abortus 544 (1 × 105 CFU/100 µl of PBS/mice) intraperitoneally. After 14 and 21 days post-challenge, three mice from each group were euthanized; spleens were removed aseptically, weighed and homogenized with 5 ml of tryptic soy broth (TSB) using sterile sand. A 10-fold serial dilution of spleen lysate was prepared in TSB and 10 µl from each dilution was plated in triplicate. For B. abortus S-19 vaccinated mice, dilution was made on TSA with 0.1% erythriotol for differentiating B. abortus S-19 and B. abortus 544. All the plates were incubated at 37°C with 5% CO2. After three days incubation, colonies were counted and expressed as log10 CFU per spleen.
Statistical analysis
The data were analysed and expressed as the mean ± SD of two experiments. Results of iELISA were analyzed by ANOVA and Dunnet’s and Tukey’s post hoc test using Prism 3.0 (Graph Pad, CA, USA) software. One way analysis of variance and Dunnet’s post hoc test using SPSS version 10.0 was used to detect significance between groups for SI, cytokine production and log CFU.
Results
SDS-PAGE and immunoblot analysis of recombinant OMP28
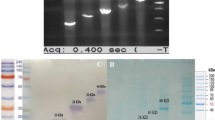
To obtain purified recombinant protein, OMP28 gene encoded polypeptide was expressed in E. coli using pPRO expression vector system. E. coli harboring pPRO-OMP28 construct was induced for expression and the expressed rOMP28 protein was purified by affinity chromatography with Ni+-NTA column. The SDS-PAGE analysis of the cell lysate and various elutes showed the expression of expected 28 kDa recombinant protein (Fig. 1). The dialyzed protein was measured to 0.5 mg/ml. In order to determine the antigenicity and specificity of the purified rOMP28, Western Blot analysis was performed using antigen specific hyperimmune sera raised in rabbit which illustrate the specific interaction between anti rOMP28 sera and purified rOMP28.
Humoral immune response
The titre of serum IgG specific to rOMP28 was determined by iELISA in pooled serum samples of five mice vaccinated with rOMP28 + CpG (group 1), rOMP28 (group 2), CpG alone (group 3), PBS control (group 4) and B. abortus S19 (group 5). Mice vaccinated with CpG adjuvanted rOMP28 and rOMP28 developed detectable antibodies to r- OMP28 two weeks post-vaccination (PV) which increased sharply after booster vaccination (Fig. 2). The OD unit of IgG in both groups was significantly higher (P < 0.001) than control group (group 4) with a peak on 4 week PV that maintained for one week and declined sharply on 6 week PV (Fig. 2). It was also noted that the IgG response to group 1 was higher (P < 0.001) than group 2 mice. However, B. abortus S19 vaccinated mice showed increase in OD from 3 week PV reaching peak on 5th week PV compared to control mice given (P < 0.001). The antibody production by the later group of mice was significantly lower compared to mice of group 1 and 2.
Comparison r-OMP28 specific antibody responses in pooled sera of mice vaccinated (intramuscular) with r-OMP28+CpG (G1), rOMP28 (G2), CpG (G3), PBS control (G4) and B. abortus S19 (G5). A booster was given to mice of all groups with respective preparations. Mice of group 5 inoculated with B. abortus S19 did not receive any booster. Pooled serum from 5 mice bled individually at weekly interval were used for detection of antibodies specific to purified r-OMP28 by indirect ELISA. Each serum sample was tested in duplicate. Neither CpG nor PBS control induced detectable antibodies responses to purified Brucella r-Omp28. OD unit represents reciprocal dilution of serum multiplied by A492 at that dilution
Lymphocyte proliferation response
The cells were stimulated with rOMP28 to evaluate the proliferative response of the protein. After 1 week post-booster, the spleenocytes from mice of group 1(rOMP28 + CpG) and group 5 (B. abortus S19) showed significant proliferative response to rOMP28 compared to group 4 (PBS), whereas the mice of group 2 (rOMP28) and group 3 (CpG) did not show any significant proliferative response to rOMP28 (Fig. 3a). However, the response was highest in group 5 (P < 0.01).
Comparison of SI of proliferative response of mice vaccinated with r-Omp28+CpG, r-Omp28, CpG, PBS control and B. abortus S19 at 1 week (a) and 3 week (b) post booster. Spleenocyte from all vaccinated group were cultured in RPMI complete media and stimulated with r-Omp28 (1 µg/ml). The data are average of SI of two individual mice from each group after 72 h
After 3 weeks of booster immunization, the spleenocytes from group 1, 2 and 5 showed significant proliferative response to rOMP28 (Fig. 3b). Again, the mice of group 5 showed highest (P < 0.01) proliferation than other groups of mice. The spleenocytes from the experimental groups (1, 2 and 5) and control groups (3 and 4) had very similar proliferative response to the mitogen ConA throughout the study.
Detection of gamma-interferon (IFN-γ) and interlukin-4(IL-4) level in immunized mice
The culture supernatant of spleenocyte culture at 7 weeks PV of the mice from each group was used to estimate the level of IFN-γ and IL-4 production following in vitro stimulation with rOMP28. The CpG vaccinated mice were found to express the highest level of IFN-γ (1,287.89 pg/ml) production followed by mice vaccinated with B. abortus S19 (625.05 pg/ml) after stimulation with rOMP28. In the present study, rOMP28 appeared to down regulate the IFN-γ production since the mice of group 2 vaccinated with rOMP28 alone produced lowest level of IFN-γ (Fig. 4a). This low production of IFN-γ was statistically significant (P < 0.05) from all other groups including the control group. However, addition of CpG with rOMP28 up-regulated IFN-γ production supported by the significantly higher (P < 0.05) IFN-γ production by mice of group 1 compared to group 2.
Quantitative estimation of lymphokines IFN-γ (a), IL-4(b) secreted by spleenocytes upon in vitro stimulation with r-OMP28 by sandwich ELISA. Spleen cells (2 × 105/ml) from mice vaccinated with rOMP-28+CpG (Gr 1), rOMP28 (Gr 2), CpG (Gr 3), PBS control (Gr 4) and B. abortus S19 (Gr5) were stimulated with RPMI1640 and r-OMP28 (1 μg/ml) for 72 hrs. Each bar represents the geometric mean ± S.D. (error bar) of the responses in spleen cells from 2 individual mice from each group
The level of IL-4 in B. abortus S19 vaccinated mice was highest compared to other groups (Fig. 4b). However, rOMP28 and CpG produced higher IL-4 (P < 0.01) compared to group 1 (rOMP28+CpG) and group 4 (PBS control).
Protection against B. abortus 544 challenge in immunized mice
To determine the protective efficacy of rOMP28, immunized mice were sacrificed on days 14 and 21 post-challenge. Protection was defined as a significant reduction in the number of bacteria in the spleen from immunized mice compared to the control mice receiving PBS. The vaccine efficacy was calculated as the log units of protection. When log10 CFU of B. abortus 544 from mice spleen of each group was determined at 2 week post challenge, maximum clearance was observed in mice of group 5 (P < 0.05) followed by group 1 (Table 1). No significant difference was seen between the groups 2, 3 and 4. The highest log unit of protection (2.349) was given by B. abortus S19 followed by rOMP28 + CpG immunized mouse. Similar trends of protection were observed at 3 weeks post challenge with highest (P < 0.05) bacterial clearance of 2.508 log unit in S19 vaccinated mice followed by the rOMP28 + CpG combination (Table 2).
Discussions
Vaccination is considered as the only method of brucellosis control for the countries with a high incidence. Although different types of live attenuated vaccines are available for control of animal brucellosis with certain drawbacks (Mc Donagh and Ficht 2006), newer strategies are needed to prevent brucellosis while avoiding the disadvantages of the currently used live vaccines. An attractive approach is the development of subunit vaccine. The protective immunity against brucellosis depends more on Th1 subset of T lymphocytes with a major role in the induction of cell-mediated immune responses. Therefore, the identification of major immunodominant antigen plays an important role in the development of effective and safer vaccine against brucellosis.
Although several antigens such as r-P39, bacterioferritin, L7/L12, GroEs, Gro EL, UvrA, Cu-Zn SOD and different OMPs (OMP31, OMP16, OMP25) has been tested for protection against experimental infection (Oliveira and Splitter 1996; Oliveira et al. 1996; Cassataro et al. 2005a; Cloeckaert et al. 1990; He et al. 2002; Cloeckaert et al. 2002), until now, significant protective activity has been identified against B. abortus for only a few purified antigens such as the L7/L12 ribosomal proteins (Oliveira and Splitter 1996), Cu-Zn SOD (Tabatabai and Pugh 1994), a 22.9 kDa protein (Tabatabai and Pugh 1994), the cytoplasmic protein P39 (Al-Mariri et al. 2001), lumazine synthase (Velikovsky et al. 2003), and r-Omp31 (Cassataro et al. 2005a). This warrants the evaluation of other purified antigens including rOMP28 for its protective propensity.
The present study demonstrates the induction of both humoral and cellular immune response and production of IFN-γ and IL-4 in the immunized mice. High serum IgG response (p < 0.05) was observed in mice of group 1 and 2 receiving rOMP28 with and without CpG, respectively. A comparatively low IgG titre in B. abortus S-19 vaccinated mice was observed, which might be due to steric hinderence of the OMP accessibility because of the presence of long lipopolysaccharide (LPS) ‘O’ side chain on smooth B. abortus S-19 as reported earlier (Cloeckaert et al. 1990; Ferrer et al. 2004). It is interesting to observe the delayed response of IgG in mice vaccinated with B. abortus S-19 (group 5) compared to the mice of group 1 and 2. Although it is difficult to explain, but the reason may be the complex nature of the live B. abortus S-19 vaccine compared to the rOMP28 (brucella is a B cell mitogen) (Schurig et al. 2002) or it might be because of the fact that rOMP28 was used as antigen to coat the ELISA plates for estimation of IgG titre. However, the significant difference (P < 0.001) in IgG titre between group 1 and 2 (Fig. 2) than the standard vaccine (B. abortus S-19) group indicates the high immunogenicity of the rOMP28 and the adjuvant effect of CpG as demonstrated earlier (Berry et al. 2004; Klinman et al. 2004; Al-Mariri et al. 2001).
Considering the intracellular nature of the pathogen, we also studied the cellular counterpart of the host immune system. The induction of cell mediated immune (CMI) response after immunization with antigens was evaluated by measuring T cell proliferation response by in vitro stimulation of spleenic cells with purified recombinant rOMP28 by MTT colorimetric method.
Antigen driven proliferative response, one week post booster, was higher (p < 0.01) in mice immunized with rOMP28 + CpG and B. abortus S-19 compared to control group (Fig. 3a). However, mice groups receiving rOMP28 alone (group 2) and CpG alone (group 3) did not show proliferation (P < 0.01). The ability of rOMP28 to mount cellular immune response is supported by rOMP28 stimulated proliferative response in B. abortus S-19 immunized mice in the present study. While co-administration of CpG with rOMP28 resulted in a significant (P < 0.05) increase in the proliferation but rOMP28 alone failed to induce lymphoproliferation, indicated up-regulation of CMI response by CpG (Al-Mariri et al. 2001; Klinman et al. 2004). Spleenocyte proliferation in B. abortus immunized mice with a fraction containing 26 kDa and 28 kDa protein was reported by Zhan et al. (1993), however, the extent of role of the later in proliferation remained unclear. Furthermore, 3 weeks post booster the blastogenic response (SI) was maximum in B. abortus S19 inoculated mice suggesting it a persistent inducer of cellular immunity. The present study demonstrates rOMP28 decreases IFN-γ production (Fig. 4a), an important cytokine for resistance against intracellular pathogens (Jacques et al. 2007) including Brucella (Spera et al. 2006; Luo et al. 2006). However, combination of rOMP28 with CpG, increased (p < 0.01) the production of IFN- γ. In the present study CpG inoculation to mice induced higher IFN- γ production (Fig. 4a) following in vitro stimulation with rOMP28. The reason for the suppressed IFN- γ production, compared to group 3 and 4, therefore, by the mice of group 1 receiving rOMP28 with CpG is not clear. We also failed to trace reports in the available literature to corroborate the present observation. This needs further study so as to elucidate the underlying mechanism that might be helpful in better modulation of host immune response by using CpG as adjuvant. The significant induction of IFN-γ production in B. abortus S-19 immunized mice could occur due to the live attenuated nature of the B. abortus S-19 vaccine strain comprising all the components and it is quite possible that the rOMP28 may act synergistically with some other molecule of Brucella leading to up-regulation of IFN-γ. Induction of a strong IFN-γ response along with relative predominance of IgG2a antibodies (data not shown) in the B. abortus S-19 immunized mice suggests induction of Th1 type of immune response by live vaccine. The IL-4 production was highest (p < 0.05) in B. abortus S-19 immunized group followed by group 2, 3 and 1, respectively (Fig. 4b). Production of IFN-γ and IL-4 along with IgG indicates a mixed response by B. abortus S-19 vaccine strain that corroborate to the findings of Yang et al. (2005).
Finally, to establish vaccine potential of rOMP28, we performed protection studies measured in terms reduction of bacterial burden in spleen of the immunized mice following challenge with B. abortus 544. The mice inoculated with B. abortus S-19 showed highest protection of 2.349 and 2.508 log units at 2 and 3 weeks post challenge, respectively, followed by mice vaccinated with rOMP28 along with CpG. On the contrary, mice inoculated with either rOMP28 or CpG did not show significant protection compared to control mice (Table 1 and 2).
Although, CpG induced cytokines have been reported to elicit protection against intracellular pathogens such as Listeria monocytogenes and Leishmania major (Krieg et al. 1998; Al-Mariri et al. 2001), it provided no or non-significant protection in the present study in CpG immunized mice challenged with B. abortus 544. The possible reason for this might be the route of administration or probably the longer interval between the booster and challenge (Al-Mariri et al. 2001). However, significant (P < 0.05) protection was observed, when CpG was co-administered with rOMP28 which was lower than other antigens like P39 (Al-Mariri et al. 2001) and rOMP31 (Cassataro et al. 2005a) reported earlier. Down regulation of IFN-γ could be the reason for the lower protection conferred by rOMP28, a critical cytokine for resistance against Brucella. This can be substantiated by the recent report of Spera et al. (2006) that a B-lymphocyte mitogen of B. abortus acts as a virulence factor for persistence of Brucella infection. Whether the rOMP28, used in the present study, too, has the same potential to act as another B-lymphocyte activator leading to persistence of Brucella infection through early modulation of immune response is yet to be conclusively established.
In conclusion, our result indicates that rOMP28 is good immunogen inducing humoral and cellular immune response however, it failed to protect brucellosis in challenged mice. Moreover, rOMP28 of Brucella when adjuvanted with CpG ODN induced Th1 biased immune response with enhanced production of IFN-γ, it failed to offer the level of protection similar to the live attenuated B. abortus S-19 vaccine against B. abortus 544 challenge.
References
Al-Mariri A, Tibor A, Mertens P, DeBolle X, Michel P, Godefroid J, Walravens K, Letesson JJ (2001) Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect. Immun 69: 4816–4822.
Bachrach G, Banai M, Bradstein S, Hoida G, Genizi A, Bercovier H (1994) Brucella ribosomal protein L7/L12 is a major component in antigenicity of brucellin INRA for delayed-type hypersensitivity in Brucella sensitized guneia pigs. Infect Immun 62:5361.
Berry LJ, Hickey DK, Skelding KA, Bao S, Rendina AM, Hansbro PM, Gockel CM, Beagley KW (2004) Transcutaneous immunization with combined cholera toxin and CpG adjuvant protects against Chlamydia muridarum Genital tract infection. Infect. Immun 72: 1019–1028.
Bhattacharjee AK, Izadjoo MJ, Zollinger WD, Nikolich MP, Hoover DL (2006) Comparison of protective efficacy of subcutaneous versus impanasal immunization of mice with a Brucella melitensis lipopolysaccharide subunit vaccine. Infect. Immun 74: 5820–5825.
Brooks-Worrell BM, Splitter GA (1996) Antigens of Brucella abortus S-19 immunodominant for bovine lymphocytes as identified by one and two dimensional cellular immunoblotting. Infect. Immun 60: 2459.
Cassataro JK, Pasquevich L, Bruno JC, Wallach CA, Fossati, Baldi PC (2004) Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough Brucellae. Clin. Diagn. Lab. Immunol 11: 111–114.
Cassataro J, Estein SM, Pasquevich KA, Velikovsky CA, Barrera S, Bowden R, Fossati CA, Giambartolomei GH (2005a) Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27 amino acid synthetic peptide elicits a CD4+ T Helper 1 response that protects against Brucella melitensis infection. Infect. Immun 73: 8079–8088.
Cassataro J, Velikovsky CA, Barrera S et al. (2005b) A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against Brucella melitensis and Brucella ovis infection by eliciting a specific cytotoxic response. Infect. Immun 73:6537–6546.
Chaudhuri P, Kumar SV, Prasad R, Srivastava SK, and Yadav MP (2005) Cloning and sequencing of 28 kDa outer membrane proein gene of brucella melitensis Rev 1. Ind.J Expt. Biol 43: 838–840.
Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV (1997) CpG oligodeoxynucleotides acts as adjuvants that switch on T helper1 (Th1) immunity. J. Exp. Med 186:1623–1631.
Cloeckaert A, Wergifosse PDE, Dubray G, Limet SN (1990) Identification of seven surface exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labelling for electron microscopy and enzyme linked immunosorbant assay. Infect. Immun 58: 3980–3987.
Cloeckaert A, Vizcaino N, Paquet JY, Bowden RA, Elzer PH (2002) Major outer membrane proteins of Brucella spp. past, present and future. Vet. Microbiol 90: 229–247.
Corbel MJ (1997a) Brucellosis an overview. Emerg. Infect. Dis 3: 213–221.
Corbel MJ (1997b) Vaccines against bacterial zoonoses. J. Med. Microbiol 46: 267–269.
Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM (1999) Bacterial DNA containing CpG motif stimulates lymphocyte-dependemt protection of mice against lethal infection with intracellular bacteria. J. Immunol 162: 2291–2298.
Ferrer MD, Leon L, Nielsen K et al. (2004) Antibody response and antigen specific gamma interferon profiles of vaccinated and unvaccinated pregnant sheep experimentally infected with Brucella melitensis. Vet. Microbiol 100: 219–231.
He Y, Vemulapalli R, Schurig GG (2002) Recombinant Ochrobacterium anthropi expressing Brucella abortus Cu, Zn superoxide dismutase protects mice against B. abortus infection only after switching of immune response to Th 1 type. Infect. Immun 70: 2535–2543.
Henk L, Smits LH, Cutler JS (2004) Contribution of biotechnology to the control and prevention of brucellosis in Africa. Afr J Biotechnol 3:631–636.
Jacques I, Verger JM, Laroucau K, Grayon M, Vizcaino N, Peire A, Cortade F, Carreras F, Guilloteau LA (2007) Immunological responses and protective efficacy againt B. melitensis induced by bp 26 and Omp31 B. melitensis Rev.1 deletion mutants in sheep. Vaccine 25: 794–805.
Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC (1998) Activation of cutaneous dendritic cells by CpG containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol 161: 3042–3049.
Janeway CA, Carding S, Jones B et al. (1998) CD4+ T cells: specificity and function. Immunol Rev 101:39–80.
Klinman DM, Currie D, Gursel I, Verthelyi D (2004) Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev 199: 201–216.
Kovarik JP, Bozzotti L, Love-Homan M, Pihlgren HL, Davis PH, Lambert AM, Krieg, Siegrist CA (1999) CpG oligonucleotides can circumvent the TH2 polarization of neonatal responses to vaccine but may fail to fully redirect TH2 responses established by neonatal priming. J. Immunol 162:1611–1617.
Krieg AM (1999) CpG DNA: a novel immunomodulator. Trends Microbiol 7:64–65.
Krieg AM (2000) The role of CpG motif in innate immunity. Curr. Opin. Immunol 12: 35–43.
Krieg AM, Yi AK, Schorr J, Davis HL (1998) The role of CpG dinucleotides in DNA vaccine. Trend. Microbiol 6: 23–27.
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680.
Lindler LE, Hadfield TL, Tall BD, Snellings NJ, Rubin FA, Van De Verg LL, Hoover D, Warren RL (1996) Cloning of a Brucella melitensis Group 3 antigen encoding Omp28, a protein recognized by the humoral immune response during human brucellosis. Infect. Immun. 64: 2490–2499.
Luo D, Ni B, Li P, Shi W, Zhang S, Han Y, Mao L, He Y, Wu Y, Wang X (2006) Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp 16 gene of Brucella abortus in BALB/c mice. Infect. Immun 74: 2734–2741.
Mallick AI, Singha H, Chaudhuri P, Nadeem A, Khan SA, Dar KA, Owais M (2007) liposomised recombinant ribosomal L7/L12 protein protects BALB/c mice against Brucella abortus 544 infection. Vaccine 25: 3692–3704.
Mc Donagh MMK. Ficht TA (2006) Evaluation of protection afforded by B. abortus and B. melitensis unmarked deletion mutants exhibiting different rates of clearance of BALB/c mice. Infect. Immun 74:4048–4057.
Mosmann TR (1983) A rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assay. J. Immunol. Method 65: 55–63.
Munoz PM, Estevan M, Marin CM et al. (2006) Brucella outer membrane complex-loaded microparticles as a vaccine against Brucella ovis in rams. Vaccine 24:1897–1905.
Oliveira SC, Splitter GA (1994) Subcloning and expression of Brucella abortus L7/L12 ribosomal gene and T lymphocyte recognition of the recombinant protein. Infect Immun 62:5201–5204.
Oliveira SC, Splitter GA (1996) Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959–962.
Oliveira SC, Zhu Y, Splitter GA (1992) Sequences of the rpIJL operon containing the L10 and L7/L12 gene from Brucella abortus. Gene 140:137.
Oliveira SC, Zhu Y, Splitter GA (1994) Recombinant L7/L12 protein and gamma irradiated Brucella abortus induces T helper 1 subset response from murine CD4+ T cells. Immunol 83:659–664.
Oliveira SC, Harms JS, Banai M, Splitter GA (1996) Recombinant B. abortus protein that induce proliferation and gamma interferon secretion by CD + 4 cells from Brucella vaccinated mice and delayed hypersensitivity in sensitized guinea pigs. Cell. Immunol 172:262–268.
Onate AA, Vemulapalli R, Andrews E, Shuring GG, Boyle S, Folch H (1999) Vaccination with live Escherichia coli expressing Brucella abortus Cu-Zn SOD protects mice against virulent Brucella abortus. Infect. Immun 67:986–988.
Paranavitana C, Elzbieta Z, Mina I, David H (2005) Interferon-γ associated cytokines and chemokines produced by spleen cells from Brucella- immune mice. Cytokine 30: 86–92
Schurig GG, Sriranganathan N, Corbel MJ (2002) Brucellosis vaccine: past, present and future. Vet. Microbiol 90: 479–496.
Spera JM, Ugalde JE, Mucci J, Comerci DJ, Ugalde RA (2006) A B-lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc. Natl. Acad. Sci 103: 16514–16519.
Splitter G, Oliveira S, Carey M, Miller C, Ko J, Covert J (1996) T lymphocyte mediated protection against facultative intracellular bacteria. Vet. Immunol. Immunopathol 54: 309–19.
Tabatabai LB, Pugh GW Jr (1994) Modulation of immune responses in BALB/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12:919–924.
Towbin U, Stawnelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications PNAS, USA76: 4350.
Velikovsky CA, Goldbaun FA, Cassataro J, Estein S, Bowden RA, Bruno L, Fossati CA, Giambartolomei H (2003) Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challanged with B. abortus 544 independently of the adjuvant formulation used. Infect. Immun 71: 5750–5755.
Vemulapalli R, He Y, Cravero S, Sriranganathan N, Boyle SM, Schurig GG (2000) Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect. Immun 68:3286–3289.
Wang X, Bao M, Wan M, Wei H, Wang L, Yu H, Zhang X, Yu Y, Wang L. A (2008) CpG oligodeoxynucleotide acts as a potent adjuvant for inactivated rabies virus vaccine. Vaccine 26:1893–1901.
Yang X, Hudson M, Walters N, Bargatze RF, Pascual DW (2005) Selection of protective epitopes for B. melitensis by DNA vaccination. Infect. Immun 73: 7297–7303.
Zhan Y, Yang J, Cheers C (1993) Cytokine response of T-cell subsets from B. abortus infected mice to soluble brucella proteins. Infect. Immun 61: 2841–2847.
Acknowledgements
We are thankful to our mentor Dr D. K Singh for his guidance and support to complete the study. We are also thankful to the Director, IVRI for providing essential requirement and financial support and Dr. P. Chaudhuri, Animal Biotechnology division, IVRI for his help and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaushik, P., Singh, D.K., Kumar, S.V. et al. Protection of mice against Brucella abortus 544 challenge by vaccination with recombinant OMP28 adjuvanted with CpG oligonucleotides. Vet Res Commun 34, 119–132 (2010). https://doi.org/10.1007/s11259-009-9337-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-009-9337-x