Abstract
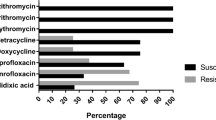
Eleven cattle farms, 8 layer farms, 7 broiler farms and 30 broiler meat samples were investigated in south-eastern Italy throughout 2003 to evaluate the prevalence, the molecular type and antimicrobial resistance of thermophilic Campylobacters. A total of 398 samples were analysed. One Campylobacter isolate for each positive faecal swab and three isolates per positive broiler meat sample were selected for further analysis. Multiplex PCR was performed for species-level identification and PCR-RFLP of the flagellin A gene for genotyping. Resistance to 14 antimicrobials was studied in 188 Campylobacter isolates. Prevalence of campylobacters was high both on farms (100%) and in food samples (73%). On 4/11 cattle farms and on 10/15 poultry farms more than one species was isolated. The presence of more than one genotype was found on 8/11 cattle farms, on 10/15 poultry farms and in 8/22 Campylobacter-positive food samples. High rates of resistance to quinolone were observed: 9/31 (29%) C. jejuni bovine isolates, 4/22 (18%) C. jejuni poultry isolates, and 14/26 (54%) C. coli poultry isolates. Resistance to sulphamethoxazole-trimethoprim was also observed frequently: 18/26 (69%) of the avian C. coli strains, 25/31 (80%) of the C. jejuni strains isolated from poultry and 15/22 (68%) of those isolated from cattle were resistant. There was a significant difference between the rate of resistance to macrolides of C. coli and C. jejuni isolated in poultry, which amounted to 23% and 3%, respectively. This study provided data on the prevalence and antimicrobial resistance of thermophilic campylobacters in south-eastern Italy and confirmed that flaA-typing is an efficient tool to study the epidemiology of Campylobacter strains in short-term investigations.
Similar content being viewed by others
Abbreviations
- PCR-RFLP:
-
polymerase chain reaction–restriction fragment length polymorphism
- bp:
-
base pair(s)
References
Aarestrup, F.M. and Wegener, H.K., 1999. The effects of antimicrobial usage in food animals on the development of antimicrobial resistance of importance in humans in Campylobacter and Escherichia coli. Microbes and Infection, 1, 639–644
Aarestrup, F.M., Mielsen, E.M., Madsen, M. and Engberg, J., 1997. Antimicrobial susceptibility patterns of thermophilic Campylobacter spp. from humans, pigs, cattle, and broilers in Denmark. Antimicrobial Agents and Chemotherapy, 41, 2244–2250
Adler-Mosca, H., Lüthy-Hottenstein, J., Martinetti Lucchini, G., Burnens, A. and Altwegg, M., 1991. Development of resistance to quinolones in five patients with campylobacteriosis treated with norfloxacin or ciprofloxacin. European Journal of Clinical Microbiology and Infectious Diseases, 10, 953–957
Bager, F., (ed.), Danmap 98, 1999. Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Danish Zoonosis Centre, Copenhagen, 3
CAMPYNET website: www.svs.dk/campynet
Charvalos, E., Peteinaki, E., Spyridaki, I., Manetas, S. and Tselentis, Y., 1996. Detection of ciprofloxacin resistance mutations in Campylobacter jejuni gyrA by nonradioisotopic single-strand conformation polymorphism and direct DNA sequencing. Journal of Clinical Laboratory Analysis, 10, 129–133
Cogan, T.A., Bloomfield, S.F. and Humphrey, T.J., 1999 The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcasses in the domestic kitchen. Letters in Applied Microbiology 29, 354–358
Endtz, H.P., Mouton, R.P., Van Der Reyden, T., Ruijs, G.J., Biever, M. and Van Klingeren, B., 1990. Fluoroquinolone resistance in Campylobacter spp. isolated from human stools and poultry products. Lancet, 335, 787
Endtz, H.P., Ruijs, G.J., Van Klingeren, B., Jansen, W.H. and Van Der Reyden, T., 1991. Quinolone resistance in Campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. Journal of Antimicrobial Chemotherapy, 42, 199–208
Fahey, T., Morgan, D., Gunneburg, C., Adak, G.K., Majid, F. and Kaczmarski, E., 1995. An outbreak of Campylobacter jejuni enteritis associated with failed milk pasteurization. Journal of Infection, 31, 137–143
Figura, N., Guglielmetti, P., Zanchi, A., Signori, R., Rossolini, A., Lior, H., Russi, M. and Musmanno, R.A., 1997. Species, biotype and serogroup of Campylobacter spp. Isolate from children with diarrhoea over a ten-year period. New Microbiologica, 20, 303–310
Gaunt, P.N. and Piddock, L.J., 1996. Ciprofloxacin resistant Campylobacter spp. in humans: an epidemiological and laboratory study. Journal of Antimicrobial Chemotherapy, 37, 47–57
Ge, B., White, D.G., Mcdermott, P.F., Girard, W., Zhao, S., Hubert, S. and Meng, J., 2003. Antimicrobial-resistant Campylobacter species from retail raw meats. Applied and Environmental Microbiology, 69, 3005–3007
Gibreel, A., Sjogren, E., Kaijser, B., Wretlind, B. and Skold, O., 1998. Rapid emergence of high-level resistance to quinolones in Campylobacter jejuni associated with mutational changes in gyrA and parC. Antimicrobial Agents and Chemotherapy, 42, 3276–3278
Griffiths, P.L. and Park, R.W.A., 1990. Campylobacters associated with human diarroheal disease. Journal of Applied Bacteriology, 69, 281–301
Husmann, M., Feddersen, A., Steitz, A., Freytag, C. and Bhakdi, S., 1997. Simultaneous identification of Campylobacters and prediction of quinolone resistance by comparative sequence analysis. Journal of Clinical Microbiology, 35, 2398–2400
Jiménez, A., Velazquez, J.B., Rodriguez, J., Tinajas, A. and Villa T, G., 1994. Prevalence of fluoroquinolone resistance in clinical resistance in clinical strains of Campylobacter jejuni isolated in Spain. Journal of Antimicrobial Chemotherapy, 33, 188–190
Li, C.C., Chiu, C.H., Wu, J.L., Huang, Y.C. and Lin, T.Y., 1998. Antimicrobial susceptibilities of Campylobacter jejuni and coli by using E-test in Taiwan. Scandinavian Journal of Infectious Disease, 30, 39–42
Linton, D., Owen, R.J. and Stanley, J., 1996. Rapid identification by PCR of the genus Campylobacter and five Campylobacter species enteropathogenic for man and animals. Research in Microbiology, 147, 707–718
Linton, D., Lawson, A.J., Owen, R.J. and Stanley, J., 1997. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. Journal of Clinical Microbiology, 35, 2568–2572
Nackamkin, I., Bohachick, K. and Patton, C.M., 1993. Flagellin gene typing of Campylbacter jejuni by restriction fragment length polymorphism analysis. Journal of Clinical Microbiology, 31, 1531–1536
National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests, Ninth informational supplement, NCCL document M100-S9, (National Committee for Clinical Laboratory Standards, Wayne, PA), 1999
Newell, D.G., Shreeve, J.E., Toszeghy, M., Dominigue, G., Bull, S., Humphrey, T. and Mead, G., 2001. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abbattoirs. Applied and Environmental Microbiology, 67, 2626–2640
Nielsen, E.M. and Nielsen, N.L., 1999. Serotypes and typability of Campylobacter jejuni and Campylobacter coli isolated from poultry products. International Journal of Food Microbiology, 46, 199–205
Nielsen, E.M., Engberg, J. and Madsen, M., 1997. Distribution of serotypes of Campylobacter jejuni and C. coli from Danish patients, poultry, cattle and swine. FEMS Immunology and Medical Microbiology, 19, 47–56
On, S.L.W., Nielsen, E.M., Engberg, J. and Madsen, M., 1998. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiology and Infection, 120, 231–237
Orr, K.E., Lightfoot, N.F., Sisson, P.R., Harkis, B.A., Tveddle, J.L. and Boyd P., 1995. Direct milk excretion of Campylobacter jejuni in a dairy cow causing cases of human enteritis. Epidemiology and Infection 114, 15–24
Owen, R.J. and Leeton, S., 1999. Restriction fragment length polymorphism analysis of the flaA gene of Campylobacter jejuni for subtyping human, animal and poultry isolates. FEMS Microbiology Letters, 176, 345–350
Park, S.F., 2002. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. International Journal of Food Microbiology, 74, 177–188
Pearson A.D., Greenwood, M.H., Donaldson, J., Healing, T.D., Jones, D.M. and Shahamat, M., 2000. Continuous source outbreak of campylobacteriosis traced to chicken. Journal of Food Protection, 63, 309–314
Petersen, L., Nielsen, E.M. and On, S.L.W., 2001. Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial poultry flocks. Veterinary Microbiology, 82, 141–154
Pezzotti, G., Serafin, A., Luzzi, I., Mioni, R., Milan, M. and Perin, R., 2003. Occurrence and resistance to antibiotics of Campylobacter jejuni and Campylobacter coli in animals and meat in northeastern Italy. International Journal of Food Microbiology, 82, 281–287
Ruiz, J., Goni, P., Marco, F., Gallardo, F., Mirelis, B., De Anta, T.J. and Vila, J., 1998. Increased resistance to quinolones in Campylobacter jejuni: a genetic analysis of gyrA gene mutations in quinolone-resistant clinical isolates. Microbiology and Immunology, 42, 223–226
Sàenz, Y., Zarazaga, M., Lantero, M., Gastanares, M.J., Baquero, F. and Torres, C., 2000. Antimicrobial resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997–1998. Antimicrobial Agents and Chemotherapy, 44, 267–271
Shane, S.M., 2000. Campylobacter infection of commercial poultry. Review in Science and Techology, 19, 376–395
Smith, K.E., Besser, J.M., Hedberg, C.W., Leano, F.T., Bender, J.B. and Wicklund, J.H., 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992–1998. New England Journal of Medicine, 340, 1525–1532
Studhal, A. and Andersson, Y., 2000. Risk factors for indigenous Campylobacter infection: a Swedish case-control study. Epidemiology and Infection, 125, 269–275
Svedhem, A., Kaijser, B. and Sjogren, E., 1981. Antimicrobial susceptibility of Campylobacter jejuni isolated from humans with diarrhoea and from healthy chickens. Journal of Antimicrobial Chemotherapy, 7, 301–305
Thomas, L.M., Long, K.A., Good, R.T., Panaccio, M. and Widders, P.R., 1997. Genotypic diversity among Campylobacter jejuni isolates in a commercial broiler flock. Applied and Environmental Microbiology, 63, 1874–1877
Wang, Y., Huang, W.M. and Taylor, D.E., 1993. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrobial Agents and Chemotherapy, 37, 457–463
Wassenaar, T.M. and Newell, D.G., 2000. Genotyping of Campylobacter spp. Applied and Environmental Microbiology, 66, 1–9
Wassenaar T.M., Geilhausen B. and Newell D.G., 1998. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Applied and Environmental Microbiology, 64, 1816–1821
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parisi, A., Lanzilotta, S.G., Addante, N. et al. Prevalence, Molecular Characterization and Antimicrobial Resistance of Thermophilic Campylobacter Isolates from Cattle, Hens, Broilers and Broiler Meat in South-eastern Italy. Vet Res Commun 31, 113–123 (2007). https://doi.org/10.1007/s11259-006-3404-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-006-3404-3