Abstract
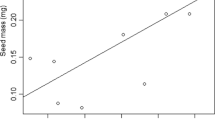
We related seed mass to summer drought survival in a Mediterranean-climate ecosystem. Previous experimental evidence linking seed mass and survival under drought is limited and at times contradictory. We tracked summer drought survival among four families/subfamilies at a restoration site in southwestern Australia. We coupled these observations with a glasshouse experiment assessing the growth and root morphology of Acacia and Eucalyptus species, with a range of seed masses, under mild and severe drought compared with a well-watered control. Summer drought survival in the field increased with seed mass across all four families/subfamilies. Seedling root biomass and length increased with seed mass consistently across five harvests over 60 days. Initial survival of seedlings in the glasshouse increased with seed mass and decreased with drought, but there was no interaction between the two. Greater absolute root investment provides a mechanism for both short and longer-term drought survival. Within-species variation in root growth may also affect the relative versus absolute survival advantage of large-seeded species. The benefits of large seed mass for establishment under environmental hazards are often considered to be temporary. Our results show that seed mass was correlated with other traits, including root length, which in turn, increased longer-term drought survival. Traits correlated with seed mass should therefore be considered in explanations of the ecological effect of seed mass variation.





Similar content being viewed by others
References
Abbott I, Dell B, Loneragan O (1989) The jarrah plant. In: Dell B, Havel JJ, Malajczuk N (eds) The jarrah forest: a complex mediterranean ecosystem. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 41–52
Baker HG (1972) Seed weight in relation to environmental conditions in California. Ecology 53(6):997–1010
Bell DT (2001) Ecological response syndromes in the flora of southwestern Western Australia: fire resprouters versus reseeders. Bot Rev 67(4):417–440
Buckley RC (1982) Seed size and seedling establishment in tropical arid dunecrest plants. Biotropica 14(4):314–315
Coomes DA, Grubb PJ (2003) Colonization, tolerance, competition and seed-size variation within functional groups. Trends Ecol Evol 18(6):283–291
Coomes DA, Rees M, Grubb PJ, Turnbull L (2002) Are differences in seed mass among species important in structuring plant communities? Evidence from analyses of spatial and temporal variation in dune-annual populations. Oikos 96(3):421–432
CSIRO (2007) Climate change in Australia: Technical Report 2007. CSIRO Publishing, Melbourne, Australia
Daws MI, Ballard C, Mullins CE, Garwood NC, Murray B, Pearson TRH, Burslem DFRP (2007) Allometric relationships between seed mass and seedling characteristics reveal trade-offs for neotropical gap-dependent species. Oecologia 154:445–454
Engelbrecht BMJ, Dalling JW, Pearson TRH, Wolf RL, Galvez DA, Koehler T, Tyree MT, Kursar TA (2006) Short dry spells in the wet season increase mortality of tropical pioneer seedlings. Oecologia 148(2):258–269
Enright NJ, Lamont BB (1989) Seed banks, fire season, safe sites and seedling recruitment in five co-occurring Banksia species. J Ecol 77:1111–1122
Falster DS, Moles AT, Westoby M (2008) A general model for the scaling of offspring size and adult size. Am Nat 172(3):299–317
Groom PK (2002) Seedling water stress response of two sandplain Banksia species differing in ability to tolerate drought. J Mediterr Ecol 3:3–9
Groom PK, Froend RH, Mattiske EM (2000) Impact of groundwater abstraction on a Banksia woodland, Swan Coastal Plain, Western Australia. Ecol Manag Restor 1(2):117–124
Guerrero-Campo J, Fitter AH (2001) Relationships between root characteristics and seed size in two contrasting floras. Acta Oecol 22(2):77–85
Herrera CM, Jordano P, Lopezsoria L, Amat JA (1994) Recruitment of a mast-fruiting, bird-dispersed tree—bridging frugivore activity and seedling establishment. Ecol Monogr 64(3):315–344
Hewitson BC, Crane RG (2006) Consensus between GCM climate change projections with empirical downscaling: precipitation downscaling over South Africa. Int J Climatol 26:1315–1337
Jurado E, Westoby M (1992a) Seedling growth in relation to seed size among species of arid Australia. J Ecol 80(3):407–416
Jurado E, Westoby M (1992b) Germination biology of selected central Australian plants. Aust J Ecol 17:341–348
Kidson R, Westoby M (2000) Seed mass and seedling dimensions in relation to seedling establishment. Oecologia 125:11–17
Klausmeyer KR, Shaw MR (2009) Climate change, habitat loss, protected areas and the climate adaptation potential of species in Mediterranean ecosystems worldwide. PLoS One 4:1–9
Kos M, Poschlod P (2010) Why wait? Trait and habitat correlates of variation in germination speed among Kalahari annuals. Oecologia 162:549–559
Lamont BB, Groom PK (2002) Green cotyledons of two Hakea species control seedling mass and morphology by supplying mineral nutrients rather than organic compounds. New Phytol 153(1):101–110
Leishman MR, Westoby M (1994a) The role of large seed size in shaded conditions—experimental-evidence. Funct Ecol 8(2):205–214
Leishman MR, Westoby M (1994b) The role of seed size in seedling establishment in dry soil-conditions—experimental-evidence from semiarid species. J Ecol 82(2):249–258
Leishman MR, Wright IJ, Moles AT, Westoby M (2000) The evolutionary ecology of seed size. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities, 2nd edn. CAB International, Wallingford, UK, pp 31–57
Lloret F, Casanovas C, Penuelas J (1999) Seedling survival of Mediterranean shrubland species in relation to root:shoot ratio, seed size and water and nitrogen use. Funct Ecol 13(2):210–216
McDonald T, Williams J (2009) A perspective on the evolving science and practice of ecological restoration in Australia. Ecol Manag Restor 10:113–125
Metz J, Liancourt P, Kigel J, Harel D, Sternberg M, Tielborger K (2010) Plant survival in relation to seed size along environmental gradients: a long-term study from semi-arid and Mediterranean annual plant communities. J Ecol 98(3):697–704
Milberg P, Lamont BB (1997) Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol 137(4):665–672
Milberg P, Perez-Fernandez MA, Lamont BB (1998) Seedling growth response to added nutrients depends on seed size in three woody genera. J Ecol 86(4):624–632
Miranda JD, Padilla FM, Lazaro R, Pugnaire FI (2009) Do changes in rainfall patterns affect semiarid annual plant communities? J Veg Sci 20(2):269–276
Moles AT, Westoby M (2004) Seedling survival and seed size: a synthesis of the literature. J Ecol 92(3):372–383
Moles AT, Westoby M (2006) Seed size and plant strategy across the whole life cycle. Oikos 113(1):91–105
Nicholls N (2006) Detecting and attributing Australian climate change: a review. Aust Meteorol Mag 55(3):199–211
Nicotra AB, Babicka N, Westoby M (2002) Seedling root anatomy and morphology: an examination of ecological differentiation with rainfall using phylogenetically independent contrasts. Oecologia 130:136–145
Paczkowska G, Chapman AR (2000) The Western Australian flora: a descriptive catalogue. Wildflower Society of Western Australia (Inc.), Western Australian Herbarium. CALM and the Botanic Gardens & Park Authority, Perth, Australia
Padilla FM, Pugnaire FI (2007) Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct Ecol 21(3):489–495
Padilla FM, Miranda JD, Pugnaire FI (2007) Early root growth plasticity in seedlings of three Mediterranean woody species. Plant Soil 296(1–2):103–113
Paz H, Martinez-Ramos M (2003) Seed mass and seedling performance within eight species of Psychotria (Rubiaceae). Ecology 84(2):439–450
Peco B, Rico L, Azcarate FM (2009) Seed size and response to rainfall patterns in annual grasslands: 16 years of permanent plot data. J Veg Sci 20:8–16
Rees M (1995) Community structure in sand dune annuals: is seed weight a key quantity? J Ecol 83:857–863
Salisbury EJ (1942) The reproductive capacity of plants. G. Bell and Sons, London, UK
Saverimuttu T, Westoby M (1996) Seedling longevity under deep shade in relation to seed size. J Ecol 84(5):681–689
Schutz W, Milberg P, Lamont BB (2002) Germination requirements and seedling responses to water availability and soil type in four eucalypt species. Acta Oecol 23(1):23–30
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York
Stock WD, Pate JS, Delfs J (1990) Influence of seed size and quality on seedling development under low nutrient conditions in 5 Australian and South-African members of the Proteaceae. J Ecol 78(4):1005–1020
Swanborough P, Westoby M (1996) Seedling relative growth rate and its components in relation to seed size: phylogenetically independent contrasts. Funct Ecol 10(2):176–184
Turnbull LA, Coomes DA, Purves DW, Rees M (2007) How spatial structure alters population and community dynamics in a natural plant community. J Ecol 95(1):79–89
Venable DL, Brown JS (1988) The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. Am Nat 131(3):360–384
Walters MB, Reich PB (2000) Seed size, nitrogen supply, and growth rate affect tree seedling survival in deep shade. Ecology 81(7):1887–1901
Wann JM, Bell DT (1997) Dietary preferences of the black-gloved wallaby (Macropus irma) and the western grey kangaroo (M. fuliginosus) in Whiteman Park, Perth, Western Australia. J R Soc West Aust 80(2):55–62
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Ann Rev Ecol Syst 33:125–159
Winn A (1985) Effects of seed size and microsite on seedling emergence of Prunella vulgaris in four habitats. J Ecol 73(3):831–840
Wright IJ, Westoby M (1999) Differences in seedling growth behaviour among species: trait correlations across species, and trait shifts along nutrient compared to rainfall gradients. J Ecol 87:85–97
Wulff RD (1986) Seed size variation in Desmodium paniculatum. 2. Effects on seedling growth and physiological performance. J Ecol 74(1):99–114
Zencich SJ, Froend RH, Turner JV, Galailitis V (2002) Influence of groundwater depth on the seasonal sources of water accessed by Banksia tree species on a shallow, sandy coastal aquifer. Oecologia 131:8–19
Acknowledgments
We thank Kellie Maher and Tim Morald for their help in the field and glasshouse and James Hallett for his comments. For access to the Gnangara restoration site we thank the Western Australian Department of Environment and Conservation, and particularly Clayton Sanders and Tracy Sonneman. LMH was supported by a Fulbright Postgraduate Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hallett, L.M., Standish, R.J. & Hobbs, R.J. Seed mass and summer drought survival in a Mediterranean-climate ecosystem. Plant Ecol 212, 1479–1489 (2011). https://doi.org/10.1007/s11258-011-9922-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-011-9922-2