Abstract
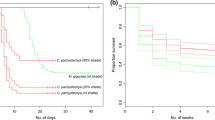
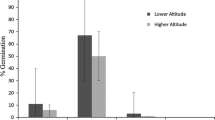
We compared requirements for seed germination and seedling establishment for Ruellia nudiflora, an invasive species in Yucatan, Mexico with those of the congeneric non-invasive R. pereducta. Germination and seedling survival rates were higher for R. nudiflora than for R. pereducta under high light. Additionally, the ranges of temperature and water potential that allow germination for R. nudiflora were much broader than those of R. pereducta. Seedlings of R. nudiflora exhibited higher survival to drought by shedding their leaves during drought, an important strategy in environments under extreme drought. Seedlings of R. nudiflora also exhibited higher extreme temperature tolerance than R. pereducta seedlings. Overall, traits exhibited by R. nudiflora such as ability to germinate under a wide range of conditions, adaptation to environmental stress and high tolerance to environmental heterogeneity during the seedling stage, have been repeatedly recognized as determinants of colonization success of invasive species in open disturbed areas.





Similar content being viewed by others
References
Ackerly D (2004) Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol Mon 74:25–44
Andrade JL (2003) Dew deposition on epiphytic bromeliads leaves: an important event in a Mexican tropical dry deciduous forest. J Trop Ecol 19:479–488. doi:10.1017/S0266467403003535
Baruch Z, Goldstein G (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121:183–192. doi:10.1007/s004420050920
Baskin CC, Baskin JM (2001) Seeds; ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego
Binggeli P (1996) A taxonomic, biogeographical and ecological overview of invasive woody plants. J Veg Sci 7:121–124. doi:10.2307/3236424
Campos-Ríos MG, Chiang F (2006) Una revision nomenclatural de los tipos de plantas de la Península de Yucatán (México). Polibotanica 22:89–149
Cervera JC, Andrade JL, Simá JL, Graham E (2006) Microhabitats, germination and establishment for Mammillaria gaumeri (Cactaceae), a rare species from Yucatan. Int J Plant Sci 167:311–319. doi:10.1086/498650
Clark JS, Lewis M, Horvath L (2001) Invasion by extremes: population spread with variation in dispersal and reproduction. Am Nat 157:537–554. doi:10.1086/319934
Daniel TF (1997) The Acanthaceae of California and the Peninsula of Baja California. Proc Calif Acad Sci 49:309–403
Didden-Zopfy B, Nobel PS (1982) High temperature tolerance and heat acclimation of Opuntia bigelovii. Oecologia 52:176–180. doi:10.1007/BF00363833
Engelbrecht BMJ, Kursar TA (2003) Comparative drought resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136:383–393. doi:10.1007/s00442-003-1290-8
Epstein E, Bloom AJ (2004) Mineral nutrition of plants: principles and perspectives. Sinauer, Sunderland, Massachusetts
Goldberg D (1987) Neighborhood competition in an old-field plant community. Ecology 68:1211–1223
Kleeman SGL, Chauhan BS, Gill GS (2007) Factors affecting seed germination of perennial wall rocket (Diplotaxis tenuifolia) in Southern Australia. Weed Sci 55:481–485. doi:10.1614/WS-06-197.1
Long RW (1977) Artificial induction of obligate cleistogamy in species hybrids in Ruellia (Acanthaceae). Bull Torrey Bot Club 104:53–56. doi:10.2307/2484665
Mack RN (1996) Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biol Conserv 78:107–121. doi:10.1016/0006-3207(96)00021-3
McAlpine KG, Drake DR (2002) The effects of small-scale environmental heterogeneity on seed germination in experimental treefall gaps in New Zealand. Plant Ecol 165:207–215. doi:10.1023/A:1022247707932
McAlpine KG, Jesson LK (2008) Linking seed dispersal, germination and seedling recruitment in the invasive species Berberis darwinii (Darwin’s barberry). Plant Ecol 197:119–129. doi:10.1007/s11258-007-9365-y
McDowell SLC (2002) Photosynthetic characteristics of invasive and non-invasive species of Rubus (Rosaceae). Am J Bot 89:1431–1438. doi:10.3732/ajb.89.9.1431
Meyer J-Y, Lavergne C (2004) Beautés fatales: Acanthaceae species as invasive alien plants on tropical Indo-Pacific islands. Divers Distrib 10:333–347. doi:10.1111/j.1366-9516.2004.00094.x
Michel BE, Radcliffe D (1995) A computer program relating solute potential to solution composition for five solutes. Agron J 87:126–130
Nobel PS, De la Barrera E (2003) Tolerances and acclimation to low and high temperatures for cladodes, fruits and roots of a widely cultivated cactus, Opuntia ficus-indica. New Phytol 157:271–279. doi:10.1046/j.1469-8137.2003.00675.x
Noble IR (1988) Plant ecology. Academic Press, London
Pons TL (1991) Induction of dark dormancy in seeds: its importance for the seed bank in the soil. Funct Ecol 5:669–675. doi:10.2307/2389487
Poorter L, Markesteijn L (2008) Seedling traits determine drought tolerance of tropical tree species. Biotropica 40:321–331. doi:10.1111/j.1744-7429.2007.00380.x
Pysek P (1998) Is there a taxonomic pattern to plant invasions? Oikos 82:282–294. doi:10.2307/3546968
Pysek P, Richardson DM (2007) Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W (ed) Biological invasions, ecological studies 193. Springer Verlag, Berlin, pp 97–125
Rejmanek M (1996) A theory of seed plant invasiveness: the first sketch. Biol Conserv 78:171–181. doi:10.1016/0006-3207(96)00026-2
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332. doi:10.1146/annurev.ecolsys.32.081501.114037
Schütz W, Milberg P, Lamont BB (2002) Seed dormancy, after-ripening and light requirements of four annual Asteraceae in south-western Australia. Ann Bot (Lond) 90:707–714. doi:10.1093/aob/mcf250
Sokal RR, Rohlf JH (1969) Biometry. Freeman and Co, New York
Standley PC, Williams LO, Gibson DN (1974) Flora of Guatemala. Bignoniaceae, Pedaliaceae, Martyniaceae, Orobanchaceae, Gesneriaceae, Lentibulariaceae, Acanthaceae. Plantaginaceae. Fieldiana Bot 24:153–466
Tripp E (2007) Evolutionary relationships within the species-rich genus Ruellia (Acanthaceae). Syst Bot 32:628–649. doi:10.1600/036364407782250625
Turner BL (1991) Texas species of Ruellia (Acanthaceae). Phytologia 71:281–299
Tyree MT, Engelbrecht BMJ, Vargas G, Kursar TA (2003) Desiccation tolerance of five tropical seedlings in Panama. Relationship to a field assessment of drought performance. Plant Physiol 132:1439–1447. doi:10.1104/pp.102.018937
Veenendaal EM, Swaine MD, Agyeman VK, Abebrese IK, Mullins CE (1996) Differences in plant and soil water relations in and around a forest gap in West Africa may influence seedling establishment and survival. J Ecol 84:83–90. doi:10.2307/2261702
Villaseñor JL, Espinosa FJ (1998) Catálogo de malezas de México. Universidad Nacional Autónoma de México y Fondo de Cultura Económica, México City
Williams DG, Black RA (1993) Phenotypic variation in contrasting temperature environments: growth and photosynthesis in Pennisetum setaceum from different altitudes on Hawaii. Funct Ecol 7:623–633. doi:10.2307/2390140
Acknowledgements
We thank Luis Salinas, Dennis Marrufo for field assistance and Luis Abdala and two anonymous reviewers for comments that improved an earlier version of the manuscript. This study was financially supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT) through a postdoctoral fellowship to JCC and as part of a grant given to VPT (SEP 2004-CO1-4658A/A1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cervera, J.C., Parra-Tabla, V. Seed germination and seedling survival traits of invasive and non-invasive congeneric Ruellia species (Acanthaceae) in Yucatan, Mexico. Plant Ecol 205, 285–293 (2009). https://doi.org/10.1007/s11258-009-9617-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-009-9617-0