Abstract
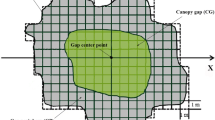
We asked the following questions regarding gap dynamics and regeneration strategies in Juniperus-Laurus forests: How important are gaps for the maintenance of tree diversity? What are the regeneration strategies of the tree species? Thirty canopy openings were randomly selected in the forest and in each the expanded gap area was delimited. Inside expanded gaps the distinction was made between gap and transition zone. In the 30 expanded gaps a plot, enclosing the gap and transition zone, was placed. In order to evaluate the differences in regeneration and size structure of tree species between forest and expanded gaps, 30 control plots were also delimited in the forest, near each expanded gap. In the 60 plots the number of seedlings, saplings, basal sprouts and adults of tree species were registered. Canopy height and width of adult individuals were also measured. The areas of the 30 gaps and expanded gaps were measured and the gap-maker identified. Juniperus-Laurus forests have a gap dynamic associated with small scale disturbances that cause the death, on average, of two trees, mainly of Juniperus brevifolia. Gap and expanded gap average dimensions are 8 and 25 m2, respectively. Gaps are of major importance for the maintenance of tree diversity since they are fundamental for the regeneration of all species, with the exception of Ilex azorica. Three types of regeneration behaviour and five regeneration strategies were identified: (1) Juniperus brevifolia and Erica azorica are pioneer species that regenerate in gaps from seedlings recruited after gap formation. However, Juniperus brevifolia is a pioneer persistent species capable of maintaining it self in the forest due to a high longevity and biomass; (2) Laurus azorica and Frangula azorica are primary species that regenerate in gaps from seedlings or saplings recruited before gap formation but Laurus azorica is able to maintain it self in the forest through asexual regeneration thus being considered a primary persistent species; (3) Ilex azorica is a mature species that regenerates in the forest.






Similar content being viewed by others
References
Abe S, Masaki T, Nakashizuka T (1995) Factors influencing sapling composition in canopy gaps of a temperate deciduous forest. Vegetatio 120:21–32
Arévalo JR (1998) Organización espacial y temporal de la Laurissilva de Anaga – Tenerife, Islas Canarias. Ph.D. Dissertation, Universidade de La Laguna
Arévalo JR, Fernández-Palacios JM (2003) Spatial patterns of trees and juveniles in a Laurel forest of Tenerife, Canary Islands. Plant Ecol 165:1–10. doi:10.1023/A:1021490715660
Arrieta S, Suárez F (2005) Spatial patterns of seedling emergence and survival as a critical phase in holly (Ilex aquifolium L) woodland recruitment in Central Spain. For Ecol Manage 205:267–282. doi:10.1016/j.foreco.2004.10.009
Attiwill PM (1994) The disturbance of forest ecosystems: the ecological basis for conservative management. For Ecol Manage 63:247–300. doi:10.1016/0378-1127(94)90114-7
Azevedo EB (1996) Modelação do clima insular à escala local – Modelo CIELO aplicado à ilha Terceira. Ph.D. Dissertation, Universidade dos Açores
Azevedo EB, Pereira LS, Itier B (1999) Modeling the local climate in island environments: water balance applications. Agric Water Manage 40:393–403. doi:10.1016/S0378-3774(99)00012-8
Borges PAV, Lobo JM, Azevedo EB, Gaspar CS, Melo C, Nunes LV (2006) Invasibility and species richness of island endemic arthropods: a general model of endemic vs. exotic species. J Biogeogr 33:169–187. doi:10.1111/j.1365-2699.2005.01324.x
Brokaw NVL (1982) The definition of treefall gap and its effect on measures of forest dynamics. Biotropica 14(2):158–160. doi:10.2307/2387750
Bruijnzeel LA (2000) Hydrology of tropical montane cloud forests: a reassessment. Land Use Water Resour Res 1:1–18
Busing RT, Fujimori T (2002) Dynamics of composition and structure in an old Sequoia sempervirens forest. J Veg Sci 13:785–792. doi:10.1658/1100-9233(2002)013[0785:DOCASI]2.0.CO;2
Carvalho LMT, Fontes MAL, Oliveira-Filho AT (2000) Tree species distribution in canopy gaps and mature forest in an area of cloud forest of the Ibitipoca Range, south-eastern Brazil. Plant Ecol 149:9–22. doi:10.1023/A:1009836810707
Choi J, Lorimer CG, Vanderwerker J, Cole WG, Martin GL (2001) A crown model for simulating long-term stand and gap dynamics in northern hardwood forests. For Ecol Manage 152:235–258. doi:10.1016/S0378-1127(00)00606-X
Coates KD (2000) Conifer seedling response to northern temperate forest gaps. For Ecol Manage 127:249–269. doi:10.1016/S0378-1127(99)00135-8
Dias E (1996) Vegetação natural dos Açores. Ecologia e sintaxonomia das florestas naturais. Ph.D. Dissertation, Universidade dos Açores
Dias E, Elias RB, Nunes V (2004) Vegetation mapping and nature conservation: a case study in Terceira Island (Azores). Biodivers Conserv 13:1519–1539. doi:10.1023/B:BIOC.0000021326.50170.66
Drobyshev IV (1999) Regeneration of Norway spruce in canopy gaps in Sphagnum-Myrtillus old-growth forests. For Ecol Manage 115:71–83. doi:10.1016/S0378-1127(98)00437-X
Drobyshev IV (2001) Effect of natural disturbances on the abundance of Norway spruce (Picea abies (L.) Karst.) regeneration in nemoral forests of the southern boreal zone. For Ecol Manage 140:151–161. doi:10.1016/S0378-1127(00)00324-8
Elias RB (2007) Ecologia das florestas de Juniperus dos Açores. Ph.D. Dissertation, Universidade dos Açores
Elias RB, Dias E (2004) Primary succession on lava domes on Terceira (Azores). J Veg Sci 15:331–338. doi:10.1658/1100-9233(2004)015[0331:PSOLDO]2.0.CO;2
Elias RB, Martins V (2006) Anthropogenic succession on Juniperus brevifolia forests in Terceira Island (Azores). Proceedings of the 5th international symposium on the fauna and flora of Atlantic Islands. Occasional publication of the Irish Biogeographical Society 9: 111–120.
França Z, Cruz JV, Nunes JC, Forjaz VH (2003) Geologia dos Açores: uma perspectiva actual. Acoreana 10(1):11–140
Gagnon JL, Jokela EJ, Moser WK, Huber DA (2004) Characteristics of gaps and natural regeneration in mature longleaf pine flatwoods ecosystems. For Ecol Manage 187:373–380. doi:10.1016/j.foreco.2003.07.002
Haggar JP (1988) The structure, composition and status of the cloud forests of Pico Island in the Azores. Biol Conserv 46:7–22. doi:10.1016/0006-3207(88)90106-1
Holwerda F, Burkard R, Eugster W, Scatena FN, Meesters AGCA, Bruijnzeel LA (2006) Estimating fog deposition at a Puerto Rican elfin cloud forest site: comparison of the water budget and eddy covariance methods. Hydrol Process 20:2669–2692. doi:10.1002/hyp. 6065
Hutchings MJ (1997) The structure of plant populations. In: Crawley MJ (ed) Plant ecology, 2nd edn. Blackwell, Oxford, pp 325–358
Imai N, Takyu M, Nakamura Y, Nakamura T (2006) Gap formation and regeneration of tropical mangrove forests in Ranong, Thailand. Plant Ecol 186:37–46. doi:10.1007/s11258-006-9110-y
Kneeshaw DD, Burton PJ (1997) Canopy and age structures of some old sub-boreal Picea stands in British Columbia. J Veg Sci 8:615–626. doi:10.2307/3237365
Kubota Y (2000) Spatial dynamics of regeneration in a conifer/broad-leaved forest in northern Japan. J Veg Sci 11:633–640. doi:10.2307/3236570
Kuuluvainen T, Syrjänen K, Kalliola R (1998) Structure of a pristine Picea abies forest in northeastern Europe. J Veg Sci 9:563–574. doi:10.2307/3237272
Lawton RO (1982) Wind stress and elfin stature in a montane rain forest tree: an adaptive explanation. Am J Bot 69(8):1224–1230. doi:10.2307/2442746
Lawton RO (1990) Canopy gaps and light penetration into a wind-exposed tropical lower montane rain forest. J For Res 20:659–667
Lawton RO, Putz FE (1988) Natural disturbance and gap-phase regeneration in a wind-exposed tropical cloud forest. Ecology 69(3):764–777. doi:10.2307/1941025
Lusk CH (1996) Stand dynamics of the shade-tolerant conifers Podocarpus nubigena and Saxegothaea conspicua in Chilean temperate rain forest. J Veg Sci 7:549–558. doi:10.2307/3236304
Manabe T, Nishimura N, Miura M, Yamamoto S (2000) Population structure and spatial patterns for trees in a temperate old-growth evergreen broad-leaved forest in Japan. Plant Ecol 151:181–197. doi:10.1023/A:1026512404110
McCarthy J (2001) Gap dynamics of forest trees: a review with particular attention to Boreal forests. Environ Rev 9:1–59. doi:10.1139/er-9-1-1
Miyadokoro T, Nishimura N, Yamamoto S (2003) Population structure and spatial patterns of major trees in a subalpine old-growth coniferous forest, central Japan. For Ecol Manage 182:259–272. doi:10.1016/S0378-1127(03)00045-8
Myers GP, Newton AC, Melgarejo O (2000) The influence of canopy gap size on natural regeneration of Brazil nut (Bertholletia excelsa) in Bolivia. For Ecol Manage 127:119–128. doi:10.1016/S0378-1127(99)00124-3
Ott RA, Juday GP (2002) Canopy gap characteristics and their implications for management in the temperate rainforest of southeast Alaska. For Ecol Manage 159:271–291. doi:10.1016/S0378-1127(01)00436-4
Pontailler J, Faille A, Lemée G (1997) Storms drive successional dynamics in natural forests: a case study in Fontainebleau forest (France). For Ecol Manage 98:1–15. doi:10.1016/S0378-1127(97)00073-X
Riéra B (1982) Observation sur les Chablis. Piste de St. Elie, Guyane. Bulletin de liaision du groupe de travail sur l’écosystème forestier guyanais. ORSTOM. Cayenne 6:165–183
Rivas-Martínez S, Díaz T, Fernández-González F, Izco J, Loidi J, Lousã M, Penas A (2002) Vascular plant communities of Spain and Portugal. Addenda to the syntaxonomical checklist of 2001. Part II. Itinera Geobot 15(2):433–922
Runkle JR (1981) Gap regeneration in some old-growth forests of the eastern United States. Ecology 62(4):1041–1051. doi:10.2307/1937003
Runkle JR (1982) Patterns of disturbance in some old-growth mesic forests of eastern North America. Ecology 63(5):1533–1546. doi:10.2307/1938878
Scharenbroch BC, Bockheim JG (2007) Impact of forest gaps on soil proprieties and processes in old growth northern hardwood-hemlock forests. Plant Soil 294:219–233. doi:10.1007/s11104-007-9248-y
Schnitzer SA, Carson WP (2001) Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology 82(4):913–919
Self S (1976) The recent volcanology of Terceira, Azores. J Geol Soc Lond 132:645–666. doi:10.1144/gsjgs.132.6.0645
Stadtmüller T (1987) Cloud forests in the humid tropics. United Nations University Press, Tokyo
Takahashi K, Homma K, Vetrova VP, Florenzev S, Hara T (2001) Stand structure and regeneration in a Kamchatka mixed boreal forest. J Veg Sci 12:627–634. doi:10.2307/3236902
Tanouchi H, Yamamoto S (1995) Structure and regeneration of canopy species in an old-growth evergreen broad-leaved forest in Aya district, southwestern Japan. Vegetatio 117:51–60. doi:10.1007/BF00033258
Taylor AH, Halpern CB (1991) The structure and dynamics of Abies magnifica forests in the southern Cascade Range, USA. J Veg Sci 2:189–200. doi:10.2307/3235951
Ulanova NG (2000) The effects of windthrow on forests at different spatial scales: a review. For Ecol Manage 135:155–167. doi:10.1016/S0378-1127(00)00307-8
van der Meer PJ (1997) Vegetation development in canopy gaps in a tropical rain forest in French Guiana. Selbyana 18(1):38–50
van der Meer PJ, Bongers F (1996) Formation and closure of canopy gaps in the rain forest at Nouragues, French Guiana. Vegetatio 126:167–179. doi:10.1007/BF00045602
van der Meer PJ, Bongers F, Chatrou L, Riéra B (1994) Defining canopy gaps in a tropical rain forest: effects on gap size and turnover time. Acta Oecol 15(6):701–714
van der Meer PJ, Sterck FJ, Bongers F (1998) Tree seedling performance in canopy gaps in a tropical rain forest at Nouragues, French Guiana. J Trop Ecol 14:119–137. doi:10.1017/S026646749800011X
Yamamoto S (1996) Gap regeneration of major tree species in different forest types of Japan. Vegetatio 127:203–213. doi:10.1007/BF00044641
Yamamoto S, Nishimura N (1999) Canopy gap formation and replacement pattern of major tree species among developmental stages of beech (Fagus crenata) stands, Japan. Plant Ecol 140:167–176. doi:10.1023/A:1009713002039
Yamashita A, Sano J, Yamamoto S (2002) Impact of a strong typhoon on the structure and dynamics of an old-growth Beech (Fagus crenata) forest, southwestern Japan. Folia Geobot 37:5–16. doi:10.1007/BF02803187
White PS, McKenzie MD, Busing RT (1985) A critique on overstory/understory comparisons based on transition probability analysis of an old growth Spruce-Fir stand in the Appalachians. Vegetatio 64:37–45. doi:10.1007/BF00033452
Zar J (1996) Biostatistical analysis, 3rd edn. Prentice Hall, New Jersey
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elias, R.B., Dias, E. Gap dynamics and regeneration strategies in Juniperus-Laurus forests of the Azores Islands. Plant Ecol 200, 179–189 (2009). https://doi.org/10.1007/s11258-008-9442-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9442-x