Abstract
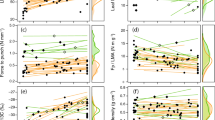
The plant functional group approach has the potential to clarify ecological patterns and is of particular importance in simplifying the application of ecological models in high biodiversity ecosystems. Six functional groups (pasture grass, pasture sapling, top-canopy tree, top-canopy liana, mid canopy tree, and understory tree) were established a priori based on ecosystem inhabited, life form, and position within the forest canopy profile on eastern Amazonian region. Ecophysiological traits related to photosynthetic gas exchange were then used to characterize such groups. The ecophysiological traits evaluated showed considerable variations among groups. The pasture grass functional group (a C4 photosynthetic pathway species) showed high instantaneous water use efficiency (A max/g s@A max), high photosynthetic nitrogen use efficiency (A max/N area), and high ratio of A max to dark respiration (A max/R d). Among the species with the C3 photosynthetic pathway, the top-canopy liana group showed the highest mean of A max/g s@A max, statistically distinct from the lowest average presented by the understory tree group. Furthermore, the pasture sapling group showed the lowest average of A max/R d, statistically distinct from the high average observed for the understory tree group. Welch-ANOVAs followed by Games–Howell post hoc tests applied to ecophysiological traits produced reasonable distinctions among functional groups, although no significant distinction was detected between the groups top-canopy tree and pasture sapling. Species distribution within the functional groups was accurately reproduced by discriminant analyses based on species averages of ecophysiological traits. The present work convincingly shows that the functional groups identified have distinct ecophysiological characteristics, with the potential to respond differently to environmental factors. Such information is of great importance in modeling efforts that evaluate the effects of dynamic changes in tropical plant communities over ecosystem primary productivity.



Similar content being viewed by others
References
Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder CR, Sandquist DR et al (2000) The evolution of plant ecophysiological traits: recent advances and future directions. BioScience 50:979–995
Carswell FE, Meir P, Wandelli EV, Bonates LCM, Kruijt B, Barbosa EM, Nobre AD, Grace J et al (2000) Photosynthetic capacity in a central Amazonian rain forest. Tree Physiol 20:179–186
Cerling TE, Ehleringer JR, Harris JM (1998) Carbon dioxide starvation, the development of C4 ecosystems, and mammalian evolution. Philos Trans Roy Soc London B 353:159–171
Chazdon RL (1988) Sunflecks and their importance to forest understorey plants. In: Begon M, Fitter AH, Ford ED, MacFadyen A (eds) Advances in ecological research. Academic Press, San Diego, pp 1–63
Chazdon RL, Pearcy RW, Lee DW, Fetcher N (1996) Photosynthetic responses of tropical forest plants to contrasting light environments. In: Mulkey SS, Kitajima K, Wright SJ (eds) Tropical forest plant ecophysiology. Chapman & Hall, New York, pp 5–55
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) Handbook for protocols for standardised and easy measurements of plant functional traits worldwide. Aust J Bot 51:335–380
da Rocha HR, Goulden ML, Miller SD, Menton MC, Pinto LDVO, de Freitas HC, Figueira AMS (2004) Seasonality of water and heat fluxes over a tropical forest in eastern Amazonia. Ecol Appl 14:22–32
Dawson TE, Chapin FS III (1993) Grouping plants by their form-function characteristics as an avenue for simplification in scaling between leaves and landscapes. In: Ehleringer JR, Field CB (eds) Scaling physiological processes Leaf to globe. Academic Press, San Diego, pp 313–319
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annu Rev Ecol Syst 18:431–451
Díaz S, Hodgson J, Thompson K, Cabido M, Cornelissen J et al (2004) The plant traits that drive ecosystems: evidence from three continents. J Veget Sci 15:295–304
Domingues TF, Berry JA, Martinelli LA, Ometto JPHB, Ehleringer JR (2005) Parameterization of canopy structure and leaf-level gas exchange for an eastern Amazonian tropical rain forest (Tapajos National Forest, Para, Brazil). Earth Interact 9:17
Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48:609–639
Ehleringer JR, Björkman O (1977) Quantum yields for CO2 uptake in C3 and C4 plants: dependence on temperature, CO2 and O2 concentration. Plant Physiol 59:86–90
Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112:285–299
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 33:317–345
Field CB, Mooney HA (1986) The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. University Press, Cambridge, pp 25–55
Goulden ML, Miller SD, da Rocha HR, Menton MC, de Freitas HC, Figueira AMES, de Souza CAD (2004) Diel and seasonal patterns of tropical forest CO2 exchange. Ecol Appl 14:42–54
Grime JP (2001) Plant strategies, vegetation processes, and ecosystem properties. Willey, New York
Hobbs RJ (1997) Can we use plant functional types to describe and predict responses to environmental changes? In: Smith TM, Shugart HH, Woodward FI (eds) Plant functional types. Their relevance to ecosystem properties and global change. University Press, Cambridge, pp 66–90
Hodgson JG, Wilson PJ, Hunt R, Grime JP, Thompson K (1999) Allocating C-S-R plant functional types: a soft approach to a hard problem. Oikos 85:282–294
Holdridge LR (1947) Determination of world plant formations from simple climatic data. Science 105:367–368
Hubbell SP (2005) Neutral theory in community ecology and the hypothesis of functional equivalence. Funct Ecol 19:166–172
Keller M, Palace M, Hurtt G (2001) Biomass estimation in the Tapajos National Forest, Brazil. Examination of sampling and allometric uncertainties. Forest Ecol Manage 154:371–382
Körner C (1993) Scaling from species to vegetation: the usefulness of functional groups. In: Schulze E-D, Mooney HA (eds) Biodiversity and ecosystem function. Springer-Verlag, Heidelberg, pp 117–140
Leight EG Jr (1975) Structure and climate in tropical rain forest. Annu Rev Ecol Syste 6:67–85
Lloyd J, Grace J, Miranda AC, Meir P, Wong SC, Miranda BS, Wright IR, Gash JHC et al (1995) A simple calibrated model of Amazon rainforest productivity based on leaf biochemical properties. Plant Cell Environ 18:1129–1145
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning. Current knowledge and future challenges. Science 294:804–808
Marshall B, Biscoe PV (1980) A model for C3 leaves describing the dependence of net photosynthesis on irradiance. I. Derivation. J Exp Bot 31:29–39
Martinelli LA, Almeida S, Brown IF, Moreira MZ, Victoria RL, Sternberg LSL, Ferreira CAC, Thomas WW (1998) Stable carbon isotope ratio of tree leaves, boles and fine litter in a tropical forest in Rondonia, Brazil. Oecologia 114:170–179
McWilliam A-LC, Cabral OMR, Gomes BM, Esteves JL, Roberts JM (1996) Forest and pasture leaf-gas exchange in south-west Amazonia. In: Gash JHC, Nobre CA, Roberts JM, Victoria RL (eds) Amazonian deforestation and climate. John Wiley & Sons, West Sussex, pp 265–286
Meir P, Grace J, Miranda AC (2001) Leaf respiration in two tropical rainforests: constraints on physiology by phosphorus, nitrogen and temperature. Funct Ecol 15:378–387
Miller SD, Goulden ML, Menton MC, da Rocha HR, de Freitas HC, Figueira AMES, de Souza CAD (2004) Biometric and micrometeorological measurements of tropical forest carbon balance. Ecol Appl 14:114–126
Nepstad DC, Moutinho P, Dias-Filho MB, Davidson E, Cardinot G, Markewitz D, Figueiredo R, Vianna N et al (2002) The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J Geophys Res 107(D20):8085, doi:10.1029/2001JD000360
Ometto JPH, Flanagan LB, Martinelli LA, Moreira MZ, Higuchi N, Ehleringer JR (2002) Carbon isotope discrimination in forest and pasture ecosystems of the Amazon Basin, Brazil. Global Biogeochem Cycles 16(4):1109, doi:10.1029/2001GB001462
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41:421–453
Pearcy RW, Ehleringer JR (1984) Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ 7:1–13
Peng C (2000) From static biogeographical model to dynamic global vegetation model: a global perspective on modelling vegetation dynamics. Ecol Modell 135:33–54
Phillips OL, Vásquez Martínez R, Arroyo L, Baker TR, Killeen T, Lewis SL, Malhi Y, Mendoza et al (2002) Increasing dominance of large lianas in Amazonian forests. Nature 418:770–774
Phillips OL, Baker TR, Arroyo L, Higuchi N, Killeen TJ, Laurance WF, Lewis SL, Lloyd J et al (2004) Pattern and process in Amazon tree turnover, 1976–2001. Philos Trans Roy Soc London B 359:381–407
Putz FE (1983) Liana biomass and leaf area of a “Tierra Firme” forest in the Rio Negro Basin, Venezuela. Biotropica 15:185–189
Raunkiaer C (1934) The life forms of plants and statistical plant geography. Clarendon Press, Oxford
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra. Global convergence in plant functioning. Proc Natl Acad Sci 94:13730–13734
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determinant of leaf structure and function among 23 amazonian tree species. Oecologia 86:16–24
Roberts J, Cabral OMR, de Aguiar LF (1990) Stomatal and boundary-layer conductances in an Amazonian Terra Firme Rain Forest. J Appl Ecol 27:336–353
Sá TD de A, da Costa J de PR, Roberts JM (1996) Forest and pasture conductances in southern Pará, Amazonia. In: Gash JHC, Nobre CA, Roberts JM, Victoria RL (eds) Amazonian deforestation and climate. John Wiley & Sons, West Sussex, pp 241–264
Sakai RK, Fitzjarrald DR, Moraes OLL, Staebler RM, Acevedo OC, Czikowsky MJ, da Silva R, Brait E et al (2004) Land-use change effects on local energy, water, and carbon balances in an Amazonian agricultural field. Global Change Biol 10:895–907
Schnitzer SA, Bongers F (2002) The ecology of lianas and their role in forests. Trends Ecol Evol 17:223–230
Shuttleworth WJ (1989) Micrometeorology of temperate and tropical forest. Philos Trans Roy Soc London B 324:299–334
Silver WL, Neff J, McGroddy M, Veldkamp E, Keller M, Cosme R (2000) Effects of soil texture on belowground carbon and nutrient storage in a lowland Amazonian forest ecosystem. Ecosystems 3:193–209
Solbrig OT (1993) Plant traits and adaptative strategies: Their role in ecosystem function. In: Schulze E-D, Mooney HA (eds) Biodiversity and ecosystem function. Springer-Verlag, Heidelberg, pp 97–116
Smith TM, Shugart HH, Woodward FI (1997) Plant functional types. Their relevance to ecosystem properties and global change. University Press, Cambridge
Telles E de CC, de Camargo PB, Martinelli LA, Trumbore S, da Costa ES, Santos J, Higuchi N, Oliveira RC Jr (2003) Influence of soil texture on carbon dynamics and storage potential in tropical forest soils of Amazonia. Global Biogeochem Cycles 17(2):1040, doi:10.1029/2002GB001953
Vieira S, de Camargo PB, Selhorst D, da Silva R, Hutyra L, Chambers JQ, Brown IF, Higuchi N et al (2004) Forest structure and carbon dynamics in Amazonian tropical rain forests. Oecologia 140:468–479
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
von Caemmerer S (2000) Biochemical models of leaf photosynthesis. CSIRO Publishing, Collingwood
Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O (1999) Challenging Theophrastus: a common core list of plant traits for functional ecology. J Veget Sci 10:609–620
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W et al (2005) Assessing the generality of global leaf trait relationships. New Phytologist 166:485–496
Würth MKR, Winter K, Körner C (1998) In situ responses to elevated CO2 in tropical forest understorey plants. Funct Ecol 12:886–895
Acknowledgments
We are thankful to the assistance provided by our friends at the LBA-ECO Santarém regional office. We are also in debt to J. Ometto and A. Calil for a great work on sample analyses. Financial support for this work was provided partially by a research grant from NASA LBA-Ecology to J.R.E., L.B.F., and L.A.M and partially by a research grant from NASA LBA-Ecology to J.R.E., J.A.B., and L.A.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Domingues, T., Martinelli, L. & Ehleringer, J. Ecophysiological traits of plant functional groups in forest and pasture ecosystems from eastern Amazônia, Brazil. Plant Ecol 193, 101–112 (2007). https://doi.org/10.1007/s11258-006-9251-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-006-9251-z