Abstract
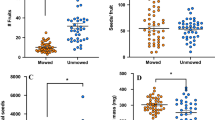
Many examples of associational resistance have been reported, in which a plant’s neighbors reduce the rate of damage by herbivores that it experiences. Despite 30 years of interest and hundreds of examples of associational resistance, we still know very little about how plants avoid their herbivores. This lack of mechanistic understanding prevents us from predicting when or where associational resistance will be important or might affect species’ distributions. I demonstrate here that the plant neighborhoods that surrounded focal mule’s ears (Wyethia mollis) individuals affected the damage they received. In particular, distance between a focal mule’s ears individual and its nearest sagebrush neighbor (Artemisia tridentata) was a good predictor of how much leaf area the mule’s ears would lose to herbivores over 2 years. Mule’s ears close to sagebrush suffered less loss than those with more distant nearest sagebrush neighbors. Mule’s ears with near sagebrush neighbors suffered half the leaf loss as mule’s ears with sagebrush experimentally removed. This associational resistance was probably not caused by sagebrush attracting or increasing populations of predators of generalist herbivores. Sagebrush is known to emit chemicals that are feeding deterrents to generalist grasshoppers and these deterrents were probably involved here. Volatile chemicals emitted by damaged sagebrush have been found to induce resistance in neighboring plants of several species. However, I found no evidence for such eavesdropping here as mule’s ears gained associational resistance from sagebrush neighbors whether or not those sagebrush neighbors had been experimentally damaged. Understanding the mechanisms responsible for associational resistance is critical to predicting where and when it will be important.



Similar content being viewed by others
References
Andow DA (1991) Vegetational diversity and arthropod population response. Annu Rev Entomol 36:561–586
Atsatt P, O’Dowd D (1976) Plant defense guilds. Science 193:24–29
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman and Hall, New York
Bertness MD, Callaway RM (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bray RO, Wambolt CL, Kelsey RG (1991) Influence of sagebrush terpenoids on mule deer preference. J Chem Ecol 17:2053–2062
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM (1997) Positive interactions in plant communities and the individualistic-continuum concept. Oecologia 112:143–149
Callaway RM et al (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Clements FE (1916) Plant succession. Carnegie Institute of Washington, Publication #242
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd ed. Lawrence Earlbaum, Hillsdale, New Jersey
Currie PO, Goodwin DL (1966) Consumption of forage by black-tailed jackrabbits on salt-desert ranges of Utah. J Wildl Manage 30:304–311
Feeny P (1976) Plant apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Biochemical interactions between plants and insects. Recent advances in phytochemistry, vol 10. Plenum, New York, pp 1–40
Gleason HA (1926) The individualistic concept of the plant association. Bull Torr Bot Club 53:7–26
Gomez-Aparicio L, Zamora R, Gomez JM, Hodar JA, Castro J, Baraza E (2004) Applying plant facilitation to forest restoration: a metaanalysis of the use of shrubs as nurse plants. Ecol Appl 14:1128–1138
Graf M (1999) Plants of the Tahoe Basin. University of California Press, Berkeley
Hamback PA, Agren J, Ericson L (2000) Associational resistance: insect damage to purple loosestrife reduced in thickets of sweet gale. Ecology 81:1784–1794
Jermy T (1984) Evolution of insect/host relationships. Am Nat 124:609–630
Jermy T (1993) Evolution of insect–plant relationships – a devil’s advocate approach. Entomol Exp Appl 66:3–12
Karban R, Baldwin IT, Baxter KJ, Laue G, Felton, GW (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125:66–71
Karban R, Baxter KJ (2001) Induced resistance in wild tobacco with clipped sagebrush neighbors: the role of herbivore behavior. J Insect Behav 14:147–156
Karban R, Maron J (2002) The fitness consequences of interspecific eavesdropping between plants. Ecology 83:1209–1213
Karban R, Maron J, Felton GW, Ervin G, Eichenseer H (2003) Herbivore damage to sagebrush induces wild tobacco: evidence for eavesdropping between plants. Oikos 100:325–333
Karban R, Huntzinger M, McCall AC (2004) The specificity of eavesdropping on sagebrush by other plants. Ecology 85:1845–1852
Karban R, Shiojiri K, Huntzinger M, McCall AC (2006) Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87:922–930
Kareiva P (1983) Influence of vegetation texture on herbivore populations: resource concentration and herbivore movement. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 259–289
Kelsey RG, Stevenson TT, Scholl JP, Watson TJ, Shafizadeh F (1978) The chemical composition of the litter and soil in a community of Artemisia tridentata spp. vaseyana. Biochem Syst Ecol 6:193–200
Kennedy PB, Doten SB (1901) A preliminary report on the summer ranges of western Nevada sheep. Nevada State University, Agricultural Experiment Station, Reno, NV Bulletin #51, 57 pp
Lortie CJ, Brooker RW, Choler P, Kikvidze Z, Michalet R, Pugnaire FI, Callaway RM (2004) Rethinking plant community theory. Oikos 107:433–438
Louda SM (1982) Limitation of recruitment of the shrub Haplopappus squarrosus (Asteraceae) by flower- and seed-feeding insects. J Ecol 70:43–53
Maron JL, Vila M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Marquis RJ (1992) The selective impact of herbivores. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens. University of Chicago Press, Chicago, pp 301–325
McAuliffe JR (1986) Herbivore-limited establishment of a Sonoran desert tree, Cercidium microphyllum. Ecology 67:276–280
McAuliffe JR (1988) Markovian dynamics of simple and complex desert plant communities. Am Nat 131:459–490
Milchunas DG, Noy-Meir I (2002) Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99:113–130
Parker VT, Yoder-Williams MP (1989) Reduction of survival and growth of young Pinus jeffreyi by an herbaceous perennial, Wyethia mollis. Am Midl Naturalist 121:105–111
Personius TL, Wambolt CL, Stephens JR, Kelsey RG (1987) Crude terpenoid influence on mule deer preference for sagebrush. J Range Manage 40:84–88
Pickford GD (1932) The influence of continued heavy grazing and of promiscuous burning on spring-fall ranges in Utah. Ecology 13:159–171
Preston CA, Laue G, Baldwin IT (2001) Methyl jasmonate is blowing in the wind but can it act as a plant–plant airborne signal?. Biochem Syst Ecol 29:1007–1023
Rausher MD (1981) The effect of native vegetation on the susceptibility of Aristolochia reticulata (Aristolochiaceae) to herbivore attack. Ecology 62:1187–1195
Shelton AM, Badenes-Perez FR (2006) Concepts and applications of trap cropping in pest management. Annu Rev Entomol 51:285–308
Tahvanainen JO, Root RB (1972) The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 10:321–346
Visser JH (1986) Host odor perception in phytophagous insects. Annu Rev Entomol 31:121–144
Whittaker RH (1967) Gradient analysis of vegetation. Biol Rev Cambridge Philos Soc 42:207–264
Wiens JA, Cates RG, Rotenberry JT, Cobb N, Van Horne B, Redak RA (1991) Arthropod dynamics on sagebrush (Artemisia tridentata): effects of plant chemistry and avian predation. Ecol Monogr 61:299–321
Young JA, Evans RA, Major J (1988) Sagebrush steppe. In: Barbour MG, Major J (eds) Terrestrial vegetation of California, 2nd ed. California Native Plant Society Special Publication #9, pp 763–769
Acknowledgments
I thank Mikaela Huntzinger and Claire Karban for help in the field, and Claire Karban, Jesse Karban, Aaron Combs, and Louie Yang for helping measuring leaf damage. Andy McCall, Louie Yang, and Truman Young improved the manuscript. I benefitted from facilities at the UC Sagehen Creek field station and Jeff Brown facilitated this research in numerous ways. This work was conducted in the Tahoe National Forest adjacent to the field station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karban, R. Associational resistance for mule’s ears with sagebrush neighbors. Plant Ecol 191, 295–303 (2007). https://doi.org/10.1007/s11258-006-9243-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-006-9243-z