Abstract
Aim
Frailty is common and is reported to be associated with adverse outcomes in patients with chronic diseases in Western countries. However, the prevalence of frailty remains unclear in individuals with chronic kidney disease (CKD) in China. We examined the prevalence of frailty and factors associated with frailty in patients with CKD.
Methods
This was a cross-sectional analysis of 177 adult patients (mean age 54 ± 15 years, 52% men) with CKD from the open cohort entitled Physical Evaluation and Adverse outcomes for patients with chronic Kidney disease IN Guangdong (PEAKING). Frailty at baseline were assessed by FRAIL scale which included five items: fatigue, resistance, ambulation, illnesses, and loss of weight. Potential risk factors of frailty including age, sex, body mass index, and daily step counts recorded by ActiGraph GT3X + accelerometer were analyzed by multivariate logistic regression analysis.
Results
The prevalence of prefrailty and frailty was 50.0% and 11.9% in patients with stages 4–5 CKD, 29.6% and 9.3% in stage 3, and 32.1% and 0 in stages 1–2. In the multivariate logistic regression analysis, an increase of 100 steps per day (OR = 0.95, 95% CI 0.91–0.99, P = 0.01) and an increase of 5 units eGFR (OR = 0.82, 95% CI 0.68–0.99, P = 0.045) were inversely associated with being frail; higher BMI was associated with a higher likelihood of being frail (OR = 1.52, 95% CI 1.11–2.06, P = 0.008) and prefrail (OR = 1.25, 95% CI 1.10–1.42, P = 0.001).
Conclusion
Frailty and prefrailty were common in patients with advanced CKD. A lower number of steps per day, lower eGFR, and a higher BMI were associated with frailty in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD), characterized by progressive loss of kidney function, represents a global public health problem due to its high prevalence and the heavy medical burden it imposes, causing more than 1.2 million deaths and 28 million years of life loss per year [1, 2].
Frailty, a clinical syndrome characterized by the decrease of physiological reserves and the increase of external dependence and disease susceptibility [3] is more common in patients with CKD [4] than that in non-CKD populations [5, 6]. Frailty has been reported to be associated with adverse outcomes including mortality [7,8,9], hospitalization [9,10,11,12], decreased renal function [13], and dialysis-related complications [14] in patients with CKD. The reported prevalence of frailty in patients with CKD varies, ranging from 7 to 73% in different populations [4]. This wide range of frailty prevalence can in part be attributed to regional differences, and differences in the severity of the disease or the tools of frailty assessment. Previous studies were performed predominantly in European and American populations [4] and in most cases in dialysis patients [14]. Little is known about the prevalence of frailty in Chinese patients with non-dialysis CKD (ND-CKD). Among several tools to evaluate the degree of frailty, the FRAIL scale (FS) has emerged as a valid and efficient tool in clinical practice [15,16,17]. The evaluation process takes less than 5 min, making it one of the most convenient tools in crowded clinical settings. However, the prevalence of frailty assessed by FS is still uncertain in Chinese patients with ND-CKD.
Considering the adverse outcomes associated with frailty, it is of great clinical and economic significance to identify individuals with high risk of frailty early in patients with CKD and to understand the associated factors of frailty in this population. Thus, this study aimed to examine the prevalence of prefrailty and frailty as defined by FS and their associated factors in Chinese patients with ND-CKD.
Methods
Study design
This is a cross-sectional analysis of adult patients from the Physical Evaluation and Adverse outcomes for patients with chronic Kidney disease IN Guangdong, China (PEAKING) study. The results of this study are reported according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [18].
PEAKING cohort and setting
The PEAKING study is an open cohort study established in 2017 and aims to investigate the level of physical activity and adverse outcomes in patients with CKD. Patients registered in the chronic disease management clinic of Guangdong Provincial Hospital of Chinese Medicine (A tertiary hospital of the region, located in Guangzhou city, the capital city of Guangdong province, China) were invited if they met the following inclusion criteria: (1) age over 18 years; (2) diagnosed as CKD with estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2 or abnormal kidney biomarkers (such as proteinuria, hematuria, etc.) for more than 90 days [19]. Patients were not invited if they met the following exclusion criteria: (1) has received or expected to receive kidney replacement therapy within 1 year; (2) pregnant or lactating women or those planning pregnancy within 1 year; (3) acute myocardial infarction or acute cerebrovascular event, acute obstructive nephropathy requiring surgery within 3 months; (4) severe arrhythmia or heart failure (New York Heart Association class grade III or above) which could not be controlled by medication; (5) active malignant tumor, decompensated cirrhosis or diseases of the hematopoietic system; (6) serious mental illness, or unable to cooperate with the investigation and treatment due to other reasons; (7) physically disability to perform physical activities;
In our study, participants were included in the analysis if they met the following: (1) complete baseline demographics and laboratory test data; (2) valid accelerometer data. The study was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (B2015-152-02), and all participants gave informed consent.
Assessment of frailty
FRAIL scale (FS) was used to evaluate the degree of frailty at baseline as recommended by the International Conference of Frailty and Sarcopenia Research (ICFSR) [20]. FS has been used in patients with all stages of CKD and demonstrated good reliability and validity [14, 21]. FS is a self-report tool and derives its name from the five domains: fatigue, resistance, ambulation, illness, and loss of weight [22]. FS score ranges from zero to 5 points. One domain represents 1 point. Those with a score more than 2 points are deemed to be with frail, 1 to 2 points are considered as prefrail stage, and those with zero points are classified as robust (Supplement Table 1).
Measurements of potential risk factors of frailty
Upon enrollment, a case report form (CRF) was used to collect baseline data of participants, including demographic data (sex, age, marital status, educational level, health insurance, working status, smoking and alcohol drinking habit). Comorbidities such as hypertension, diabetes, cardiovascular disease, stroke, chronic obstructive pulmonary disease, bronchial asthma, arthritis, malignant tumor, and osteoporosis were recorded on the CRF allowing calculation of Charlson comorbidity index (CCI) [23]. Information about the kidney disease was collected as well, including primary disease of CKD, disease duration, and kidney biopsy report if available.
Participants underwent physical examinations at baseline for anthropometric parameters including body weight and height, and waist circumference. Body mass index (BMI) was calculated accordingly and categorized as severely underweight (< 16.5 kg/m2), underweight (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), and overweight (> 23 kg/m2) [24]. In addition, their physical performance and physical activity were measured using established approaches: handgrip strength was measured as a proxy for upper limb performance using a digital handgrip dynamometer (EH101, CAMRY Sensun Weighing Apparatus Group Ltd, Guangdong, China), calculated from the largest readings of three measurements using participants’ dominant hand; the ActiGraph GT3X + accelerometers (ActiGraph, LLC Pensacola, FL, USA) were employed to evaluate daily step counts as a reliable and objective measure of physical activity. It incorporates a triaxial accelerometer that detects and records acceleration forces in different directions. These forces are then converted into activity counts, which reflect the intensity and duration of movement. This sophisticated device has gained widespread adoption by over 1500 colleges and institutions in more than 65 countries or regions worldwide [25]. They serve as precise instruments for capturing and assessing multiple facets of physical activity, encompassing not only daily step counts but also capturing the nuances of intensity, duration, and frequency of physical activity, sedentary behavior, and energy expenditure. Subjects were required to wear an accelerometer on the right hip in daytime for 9 consecutive days [26]. Data were considered valid if a participant had at least 3 days (including one non-working day) of at least 10 hours per day recorded [27]. We selected an epoch length of 60 seconds [28]. A non-wear-time was defined as an interval of at least 60 min of zero activity counts [28]. At the end of the measurement period, the accelerometer data were retrieved through the ActiLife software. Daily step counts were defined as the average of daily step counts from valid wearing days.
Finally, participants were required to have laboratory tests at baseline including the number of white blood cell, percentage of neutrophils, hemoglobin, estimated glomerular filtration rate (eGFR) estimated by CKD-EPI Creatinine Eq. (2021) [29], spot urine protein–creatinine ratio, serum potassium, total serum calcium, serum albumin, serum uric acid, serum triglyceride, and serum total cholesterol.
Statistical analysis
Data were described as percentages (%) for categorical variables and mean and standard deviation (M ± SD) or median and interquartile range [M(IQR)] for continuous variables. To test the statistical difference among groups (robust, prefrail and frail), univariate analyses were conducted using Chi-squared test for categorical variables and analysis of variance (with post hoc Tukey analysis) or Kruskal–Wallis test (with Mann–Whitney U test for post hoc analysis) for continuous variables as appropriate. Variables that showed statistical significance of P < 0.1 in the univariate analyses were included in the multivariate logistic regression analysis, which estimated the odds ratio (OR) and 95% confidence interval (CI) with the robust group as reference group. We further treated FS score as a continuous dependent variable and examined associated factors of FS score in multiple linear regression analysis with stepwise backward variable selection, incorporating the same panels of variables in the aforementioned logistic regression. A two-tailed examination with P value less than 0.05 was used as an indication of statistical significance. All analyses were conducted using the Stata 15.0 and the free statistics analysis platform.
Results
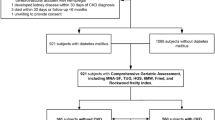
A total of 191 eligible ND-CKD adults were enrolled from the PEAKING cohort. After excluding patients with incomplete CRF data (n = 2), incomplete laboratory data (n = 2), and invalid accelerometer data (n = 10), 177 patients were included in this cross-sectional study (Fig. 1).
The mean age of participants was 54 ± 15 years and 92 (52%) were men. There were 42 (23.7%) participants with CKD stages 4–5, 54 (30.5%) with CKD stages 3a–3b, and 81 (45.8%) had CKD stages 1–2. The majority of the participants had high school or higher education (78.6%), were married (88.7%), had medical insurance (79.1%), were non-smokers (88.7%) and non-alcohol drinkers (98.3%) (Table 1). According to FRAIL assessment, 104 (58.8%) were robust, 63 (35.6%) were prefrail, and 10 (5.6%) were frail (Table 1). The prevalence of prefrailty and frailty was 50.0% and 11.9% in patients with stages 4–5 CKD, 29.6% and 9.3% in stages 3a-3b, and 32.1% and 0 in stages 1–2. CKD patients with frailty were more likely to be older (P < 0.001).
The mean BMI of participants was 22.2 ± 3.0 kg/m2, which increased as the frail degree increased (up to 90% of participants were within the normal range or overweight). The mean daily step counts of 7532 ± 3268 gradually decreased as the degree of frailty increased. As expected, frail patients were more likely having a lower level of handgrip strength (P = 0.008), less daily step counts (P < 0.001), lower eGFR (P = 0.01), and higher CCI scores (P < 0.001). Prefrail participants were more likely to have a higher CCI score (P = 0.014), higher BMI (P = 0.022), and lower eGFR (P = 0.31) (Table 2).
After univariate analyses, six potential indicators (P < 0.1) associated with frailty or prefrailty in ND-CKD patients, including age, BMI, handgrip strength, daily step counts, eGFR, and serum albumin, were included in the multivariate logistic regression analysis. Because the frailty assessment tool adopted in this study includes the module of comorbidity, comorbidity-related factors (diabetes mellitus, cardiovascular disease, and CCI) are excluded in the multivariate logistic regression analysis.
Factors associated with prefrailty in patients with ND-CKD in multivariate logistic regression
Higher BMI (OR = 1.25, 95% CI 1.10–1.42, P = 0.001) was associated with a higher likelihood of being prefrail; an increase of 5 units eGFR (OR = 0.92, 95% CI 0.87–0.99, P = 0.008) was inversely associated with being prefrail (Fig. 2).
Factors associated with frailty in patients with ND-CKD in multivariate logistic regression
An increase of 100 steps per day (OR = 0.95, 95% CI 0.91–0.99, P = 0.01) and an increase of 5 units eGFR (OR = 0.82, 95% CI 0.68–0.99, P = 0.045) were inversely associated with being frail, and higher BMI was associated with a higher likelihood of being frail (OR = 1.52, 95% CI 1.11–2.06, P = 0.008) (Fig. 3).
In the multiple linear regression analysis, with FS scores as the dependent variable, daily step counts (β = − 0.008, P < 0.001) and eGFR (β = − 0.005, P < 0.013) were associated with a lower severity of frailty, BMI (β = 0.074, P = 0.001) was associated with a higher severity of frailty among patients with ND-CKD.
Discussion
In this cross-sectional analysis of the PEAKING study on physical status and physical activity versus outcomes in patients with CKD, people with ND-CKD, especially in those with advanced CKD, prefrailty and frailty were common. Daily step counts, BMI, and eGFR were independent associated factors for frailty in this population. Our study highlights the frailty burden in patients with ND-CKD and indicates that daily steps and BMI may be used as a marker of frailty in this population.
Prefrailty was quite common (41.2%) among patients with ND-CKD while the prevalence of frailty was only 5.6%, which is slightly lower than that reported in a systematic review where the prevalence of frailty ranged from 7.0 to 42.6% among patients with ND-CKD [4]. This may be attributed to the healthier population included in this study who were all outpatient patients and most of them had relatively high eGFR. It has been reported that the prevalence of frailty increased as the eGFR decreased [13]. Therefore, we further estimated the prevalence of frailty stratified by stage of CKD and found that the prevalence of frailty was 11.9% in stages 4–5 CKD and 9.3% in stages 3a–3b CKD, which is consistent with previous study [30].
In our study, we found even small increase in daily step counts, such as 100 steps, was inversely associated with frailty in CKD patients. There are several potential explanations. On the one hand, step counts are the most common indicator of physical activity. It incorporates both light and moderate-to-vigorous physical activity and has been become a common method of assessing daily physical activity regardless of location, culture, age, and gender [31]. Increasing step counts in daily life is considered as one of the most reasonable and cost-effective approaches for reducing the risk of several diseases including frailty [32, 33]. For example, Daiki et al. found that increasing the current step count by as little as 1,000 steps/day (about 10 min of activity) may potentially prevent frailty in older adults [33]. On the other hand, the number of daily steps may correlate to skeletal muscle mass and it has been reported that frail elderly can increase skeletal muscle mass maintenance by increasing the daily step counts [34], especially in those with lower daily step counts [35]. Our finding lends support to the possibility to extend the evidence from older adults to patients with ND-CKD. Admittedly, we acknowledged that daily step counts might overlap with the ambulation domain of the FRAIL scale to some degree. However, it should be noted that the ambulation domain primarily emphasizes the dependence of frail patients on external support during walking. It does not quantify the extent of ambulation contributes to the status of frailty.
Another important finding of our study was that BMI was independently associated with a higher likelihood of both being frail and prefrail, which are in line with previously published results [10, 13]. There are several potential explanations. First, obesity, as defined by a high BMI, has been associated with decreased physical function and increased fatigue, which are key components of frailty. This decrease in physical function may be a result of the additional stress placed on the body by overweight, leading to physical inactivity and decreased muscle mass and strength [36]. Second, higher BMI is often associated with more adipose tissue, which can promote the development of inflammation, metabolism and transmission of metabolic information between different organs by secreting cytokines such as adiponectin, interleukin-6, and tumor necrosis factor, and ultimately lead to frailty [37].
On the other hand, it should be noted that most of the participants in current study had moderate or higher BMI and no one had extremely low BMI. Previous study has also found extremely low BMI was associated with higher risk of frailty in patients with CKD due to malnutrition, decreased muscle mass, and decreased physical function [38]. Therefore, it may be worthwhile to note that interventions should be directed toward moderate or higher BMI, but not extremely low, in patients with CKD.
The current study also found that eGFR was inversely associated with frailty and prefrailty. This relationship may partially be explained by the increased prevalence of associated comorbidities in patients with CKD, including cardiovascular disease, anemia, and mineral and bone disorders, all of which have been shown to contribute to an increased risk of frailty [1]. Additionally, a decline in kidney function, as indicated by a declining eGFR, is also associated with decreased physical function, another key component of frailty [39].
Our study has some strengths. We have evaluated the current prevalence of frailty and prefrailty in a representative sample of patients with ND-CKD in China. A wide range of clinical and physical parameters were investigated as potential predictors of frailty and prefrailty in this population. The use of ActiGraph GT3X+, one of the most accurate assessment tools in the field of physical activity, to record daily steps strongly enhances the validity of our approach. Nonetheless, several limitations still warrant attention. First, this study is cross-sectional in nature, and we may not be able to confirm the causal relationship between associated factors and frailty. Second, we might underestimate the prevalence of frailty since we excluded patients with physical disability. Third, data on other potential predictors of frailty such as mental health were not available in our study and prevented us from further investigating the relationship between these factors and frailty. Future studies are needed to explore the predictors of frailty among adults with CKD using a prospective longitudinal design with a larger sample size in other settings.
In conclusion, frailty and prefrailty were common in patients with ND-CKD. Daily step counts, BMI, and eGFR were associated with frailty in this population. The findings of this study may provide direction for future longitudinal studies to determine the causal relationship between these factors and frailty in patients with CKD, thereby implementing targeted potential interventions to address the risk of frailty in this population.
References
Romagnani P, Remuzzi G, Glassock R et al (2017) Chronic kidney disease. Nat Rev Dis Prim 3(1):1–24
Xie Y, Bowe B, Mokdad AH et al (2018) Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94(3):567–581
Morley JE, Vellas B, Abellan Van Kan G, et al (2013) Frailty consensus: a call to action. J Am Med Direct Assoc 14(6):392–397
Chowdhury R, Peel NM, Krosch M, Hubbard RE (2017) Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr 68:135–142
Wang XH, Mitch WE (2014) Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10(9):504–516
Nixon AC, Bampouras TM, Pendleton N, Woywodt A, Mitra S, Dhaygude A (2018) Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J 11(2):236–245
Johansen KL, Dalrymple LS, Glidden D et al (2016) Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol 11(4):626–632
Alfaadhel TA, Soroka SD, Kiberd BA, Landry D, Moorhouse P, Tennankore KK (2015) Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 10(5):832–840
McAdams-DeMarco MA, Law A, Salter ML et al (2013) Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61(6):896–901
Lee SY, Yang DH, Hwang E et al (2017) The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr 27(2):106–112
Hickson LJ, Thorsteinsdottir B, Ramar P et al (2018) Hospital readmission among new dialysis patients associated with young age and poor functional status. Nephron 139(1):1–12
Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL (2012) Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 172(14):1071–1077
Roshanravan B, Khatri M, Robinson-Cohen C et al (2012) A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis 60(6):912–921
Chao CT, Hsu YH, Chang PY et al (2015) Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology (Carlton) 20(5):321–328
Kojima G (2018) Frailty defined by FRAIL scale as a predictor of mortality: a systematic review and meta-analysis. J Am Med Dir Assoc 19(6):480–483
Thompson MQ, Theou O, Tucker GR, Adams RJ, Visvanathan R (2020) FRAIL scale: predictive validity and diagnostic test accuracy. Australas J Ageing 39(4):e529–e536
Aprahamian I, Cezar N, Izbicki R et al (2017) Screening for frailty with the FRAIL scale: a comparison with the phenotype criteria. J Am Med Dir Assoc 18(7):592–596
Vandenbroucke JP, von Elm E, Altman DG et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med 147(8):W163–W194
Inker LA, Astor BC, Fox CH et al (2014) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63(5):713–735
Dent E, Morley JE, Cruz-Jentoft AJ et al (2019) Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging 23(9):771–787
Ozturk S, Cetin DG, Cetin M et al (2022) Prevalence and associates of frailty status in different stages of chronic kidney disease: a cross-sectional study. J Nutr Health Aging 26(9):889–895
Abellan VKG, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B (2008) The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging 12(1):29–37
Hemmelgarn BR, Manns BJ, Quan H, Ghali WA (2003) Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42(1):125–132
Weir CB, Jan A (2022) BMI classification percentile and cut off points. StatPearls, Treasure Island
ActiGraph corp. Homepage. 2023. https://theactigraph.com/academic-research
LaMunion SR, Bassett DR, Toth LP, Crouter SE (2017) The effect of body placement site on ActiGraph wGT3X-BT activity counts. Biomed Phys Eng Exp 3(3):035026
Heil DP, Brage S, Rothney MP (2012) Modeling physical activity outcomes from wearable monitors. Med Sci Sports Exerc 44(1 Suppl 1):S50-60
Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M (2008) Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40(1):181–188
Inker LA, Eneanya ND, Coresh J et al (2021) New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 385(19):1737–1749
Wilhelm-Leen ER, Hall YN, Tamura MK, Chertow GM (2009) Frailty and chronic kidney disease: the third national health and nutrition evaluation survey. Am J Med 122(7):664–71.e2
Zhu Z, Chen H, Ma J, He Y, Chen J, Sun J (2020) Exploring the relationship between walking and emotional health in China. Int J Environ Res Public Health 17(23):8804
Seliger S, Weiner DE (2022) Exercise and kidney disease prevention: walk this way. Am J Kidney Dis 80(4):552–554
Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M, Group KKS (2020) Objectively measured daily step counts and prevalence of frailty in 3,616 older adults. J Am Geriatr Soc 68(10):2310–2318
Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H (2015) Mail-based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community-dwelling Japanese older adults: the INE (Intervention by Nutrition and Exercise) study. J Am Med Dir Assoc 16(8):654–660
Park H, Park S, Shephard RJ, Aoyagi Y (2010) Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol 109(5):953–961
Crow RS, Lohman MC, Titus AJ et al (2019) Association of obesity and frailty in older adults: NHANES 1999–2004. J Nutr Health Aging 23(2):138–144
Yuan L, Chang M, Wang J (2021) Abdominal obesity, body mass index and the risk of frailty in community-dwelling older adults: a systematic review and meta-analysis. Age Ageing 50(4):1118–1128
Lee SW, Lee A, Yu MY et al (2017) Is Frailty a modifiable risk factor of future adverse outcomes in elderly patients with incident end-stage renal disease? J Korean Med Sci 32(11):1800–1806
Hiraki K, Yasuda T, Hotta C et al (2013) Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol 17(2):225–231
Acknowledgements
We acknowledge the participants, the clinicians, and health care professionals in the PEAKING study
Funding
Open access funding provided by Karolinska Institute. G.S. acknowledges support from National Nature Science Foundation of China (No. 82004205), The Spring Sunshine Program of Scientific Research Cooperation, Ministry of Education of China (NO. HZKY20220109), National Administration of Traditional Chinese medicine, P.R. China (No. 2023ZYLCYJ02-18), Research Fund for Bajian Talents of Guangdong Provincial Hospital of Chinese Medicine (No. BJ2022KY11), the Science and Technology Research Fund from Guangdong provincial hospital of Chinese medicine, China (No. YN2018QL08), the Karolinska Institutet’s internal funds (No. 2020–01616; No. 2022–02044). X.Q. acknowledges support from Traditional Chinese Medicine Bureau of Guangdong Province (No. 20211194). The funding sources were not involved in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conception and design: CY, GS, FL; data acquisition: CX, JZ, RD, XL, JQ; data analysis: CY, GS; data interpretation: all authors. Each author contributed important intellectual content during manuscript drafting or revision and agreed to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including documentation in the literature if appropriate.
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors declare conflicts of interest.
Data availability
The data that support the findings of this study are available from PEAKING study upon reasonable request. The datasets used and/or analyzed during the current study are also available in the Guangdong Provincial Hospital of Chinese Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, C., Xiao, C., Zeng, J. et al. Prevalence and associated factors of frailty in patients with chronic kidney disease: a cross-sectional analysis of PEAKING study. Int Urol Nephrol 56, 751–758 (2024). https://doi.org/10.1007/s11255-023-03720-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03720-z