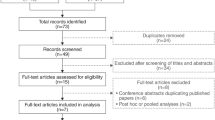
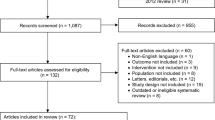
Abstract
Purpose
The efficacy and safety of vibegron, which is a novel β3-adrenoceptor agonist, need to be systematically evaluated in the treatment of overactive bladder (OAB) patients. This study aimed to assess, using meta-analytic methods, the efficacy and safety of vibegron for OAB compared with placebo or antimuscarinics, considering all available data from comparative studies.
Methods
A systematic search was performed on MEDLINE, Web of Science, and Cochrane Central Register of Controlled Trials database to identify studies from their date of inception before 15 March 2020. Meta-analysis was performed based on eligible studies.
Results
Six studies derived from four clinical trials with 4314 randomized patients were finally included in our analysis. We found that vibegron 50 mg and 100 mg were both significantly more efficacious than placebo for all efficacy outcomes. Furthermore, no significant differences were found between vibegron 50 mg or 100 mg and placebo for all the AEs assessed. In addition, the efficacy between vibegron 50 mg or 100 mg and antimuscarinics were comparable except for voided volume. Moreover, vibegron was associated with a decreased risk of dry mouth and an increased risk of nasopharyngitis versus antimuscarinics. Our study also demonstrated that the vibegron 50 mg and 100 mg were equally effective and safe across all the efficacy and AEs’ outcomes.
Conclusions
Vibegron is effective and safe for treating patients with OAB. Based on the current evidence, we recommended that the initial use of vibegron 50 mg was the optimal algorithm in the pharmacologic management of OAB.

Similar content being viewed by others
References
Stewart WF, Van Rooyen JB, Cundiff GW et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20(6):327–336
Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ (2001) How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87(9):760–766
Sexton CC, Notte SM, Maroulis C et al (2011) Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract 65(5):567–585
Chapple CR, Nazir J, Hakimi Z et al (2017) Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: a retrospective observational study in UK clinical practice. Eur Urol 72(3):389–399
Kelleher C, Hakimi Z, Zur R et al (2018) Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol 74(3):324–333
Edmondson SD, Zhu C, Kar NF et al (2016) Discovery of vibegron: a potent and selective beta3 adrenergic receptor agonist for the treatment of overactive bladder. J Med Chem 59(2):609–623
Di Salvo J, Nagabukuro H, Wickham LA et al (2017) Pharmacological characterization of a novel beta 3 adrenergic agonist, vibegron: evaluation of antimuscarinic receptor selectivity for combination therapy for overactive bladder. J Pharmacol Exp Ther 360(2):346–355
Yoshida M, Takeda M, Gotoh M, Nagai S, Kurose T (2018) Vibegron, a novel potent and selective beta3-adrenoreceptor agonist, for the treatment of patients with overactive bladder: a randomized, double-blind, placebo-controlled phase 3 study. Eur Urol 73(5):783–790
Mitcheson HD, Samanta S, Muldowney K et al (2019) Vibegron (RVT-901/MK-4618/KRP-114V) administered once daily as monotherapy or concomitantly with tolterodine in patients with an overactive bladder: a multicenter, phase IIb, randomized, double-blind, controlled trial. Eur Urol 75(2):274–282
Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN Jr (2020) International phase III, randomized, double-blind, placebo- and active-controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol. 101097JU0000000000000807
Yoshida M, Kakizaki H, Takahashi S, Nagai S, Kurose T (2018) Long-term safety and efficacy of the novel beta3-adrenoreceptor agonist vibegron in Japanese patients with overactive bladder: a phase III prospective study. Int J Urol 25(7):668–675
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269 (W264)
Qiu C, Zhao X, She L et al (2019) Baricitinib induces LDL-C and HDL-C increases in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Lipids Health Dis 18(1):54
Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The cochrane collaboration, 2011. Available from www.cochrane-handbook.org
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J (2014) A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol 66(3):529–541
Yoshida M, Takeda M, Gotoh M et al (2019) Efficacy of novel beta3-adrenoreceptor agonist vibegron on nocturia in patients with overactive bladder: a post-hoc analysis of a randomized, double-blind, placebo-controlled phase 3 study. Int J Urol 26(3):369–375
Yoshida M, Takeda M, Gotoh M et al (2020) Efficacy of vibegron, a novel beta3-adrenoreceptor agonist, on severe urgency urinary incontinence related to overactive bladder: post hoc analysis of a randomized, placebo-controlled, double-blind, comparative phase 3 study. BJU Int 125(5):709–717
La Rosa VL, Duarte de Campos da Silva T, Rosa de Oliveira A, Marques Cerentini T, Viana da Rosa P, Telles da Rosa LH (2020) Behavioral therapy versus drug therapy in individuals with idiopathic overactive bladder: a systematic review and meta-analysis. J Health Psychol 25(5):573–585
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I (2008) The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 101(11):1388–1395
Vitale SG, Capriglione S, Zito G et al (2019) Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet 299(2):299–315
Kruppa J, Kavvadias T, Amann S, Baessler K, Schuessler B (2016) Short and long-term urodynamic and quality of life assessment after nerve sparing radical hysterectomy: a prospective pilot study. Eur J Obstet Gynecol Reprod Biol 201:131–134
Funding
This article is supported by grants from 1.3.5 project for disciplines of excellence, West China Hospotal, Sichuan University (ZYGD18011, ZY2016104 and ZYJC18015).
Author information
Authors and Affiliations
Contributions
JZ: project development, data collection and management, manuscript writing, and revising; YC: data collection, data analysis; LH: data collection, and data analysis; ZW: data collection and data analysis; WK: project design and development, data interpretation, manuscript editing, and revising. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article as well as all the included studies declare that they have no conflict of interest.
Ethical standards
The study protocol is compliant with ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jian, Z., Yuan, C., Li, H. et al. Vibegron 50 mg is the optimal algorithm in the pharmacologic management of overactive bladder: outcomes from a systematic review and meta-analysis. Int Urol Nephrol 52, 2215–2221 (2020). https://doi.org/10.1007/s11255-020-02536-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02536-5