Abstract
Purpose
Peritoneal fibrosis is almost uniform feature encountered in peritoneal dialysis patients. The transition of epithelial cells to mesenchymal phenotype, neovascularization, and consequently development of peritoneal fibrosis occur due to the involvement of peritoneal membrane by various insults such as uremia itself, peritonitis attacks, and exposure to bio-incompatible peritoneal dialysis fluids. Bevacizumab is a monoclonal antihuman antibody developed against vascular endothelial growth factor and can reduce fibrosis by preventing neovascularization. There has been no study so far that demonstrates the effect of bevacizumab on peritoneal fibrosis in a rat model.
Methods
A total of 41 female Wistar albino rats were divided into six groups. The control group (C) received 0.9 % isotonic saline (2 ml/day) intraperitoneally (i.p) for 21 days. Chlorhexidine group (CH) received 15 % ethyl alcohol and 0.1 % chlorhexidine gluconate (CG) in saline (2 ml/day) i.p for 21 days. The resting group (R) received CG 2 ml/day i.p for 21 days. The bevacizumab-1 group (B1) received CG 2 ml/day i.p for 21 days and bevacizumab 2.5 mg/kg i.p as a single dose on day 21. The bevacizumab-2 group (B2) received CG 2 ml/day for 21 days and bevacizumab 2.5 mg/kg i.p on day 0 and day 21. The bevacizumab-3 group (B3) received bevacizumab 2.5 mg/kg i.p on day 0 and day 21. Peritoneal samples were taken from the left anterior abdominal wall. The thickness, vascularization, and fibrosis scores in the peritoneal samples were assessed using a light microscope.
Results
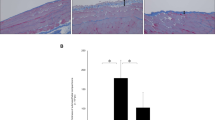
On histopathological evaluations, peritoneum thicknesses, vascularization scores, and fibrosis significantly decreased in bevacizumab groups B1 and B2.
Conclusion
Histopathologically, bevacizumab was proven to attenuate fibrotic process in experimental peritoneal sclerosis model.


Similar content being viewed by others
References
Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K (2013) Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: a marginal structural model analysis. Clin J Am Soc Nephrol 8(4):619–628
Kawaguchi Y, Saito A, Kawanishi H et al (2005) Recommendations on the management of encapsulating peritoneal sclerosis in japan 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int 25(4):S83–S95
Davies SJ (2014) Peritoneal solute transport and inflammation. Am J Kidney Dis 64(6):978–986
Morinaga H, Sugiyama H, Inoue T, Takiue K, Kikumoto Y, Kitagawa M et al (2012) Effluent free radicals are associated with residual renal function and predict technique failure in peritoneal dialysis patients. Perit Dial Int 32(4):453–461
Latus J, Ulmer C, Fritz P, Rettenmaier B, Biegger D, Lang T et al (2013) Encapsulating peritoneal sclerosis: a rare, serious but potentially curable complication of peritoneal dialysis-experience of a referral centre in Germany. Nephrol Dial Transplant 28(4):1021–1030
Krediet RT, Lindholm B, Rippe B (2000) Pathophysiology of peritoneal membrane failure. Perit Dial Int 20(4):S22–S42
Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K (1996) Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of sclerosing encapsulating peritonitis group. Am J Kidney Dis 28:420–427
Paolo N, Garosi G (1999) Peritoneal sclerosis. J Nephrol 12:347–361
Brulez HF, Verbrugh HA (1995) First-line defense mechanisms in the peritoneal cavity during peritoneal dialysis. Perit Dial Int 15(S7):S24–S33
Yaῆez-Mo M, Lara-pezzi E, Selgas R et al (2003) Peritoneal dialysis and epithelial to-mesenchymal transition of mesothelial cells. N Eng J Med 348:403–413
Seeger H, Braun N, Latus J, Alscher MD, Fritz P, Edenhofer I et al (2014) Platelet-derived growth factor receptor-β expression in human peritoneum. Nephron Clin Pract 128:178–184
Braun N, Reimold F, Biegger D, Fritz P, Kimmel M, Ulmer C et al (2009) Fibrogenic growth factors in encapsulating peritoneal sclerosis. Nephron Clin Pract 113(2):88–95
Krediet RT, Zweers MM, van der Wal AC, Struijk DG (2000) Neoangiogenesis in the peritoneal membrane. Perit Dial Int 20(2):S19–S25
Williams JD, Craig KJ, Topley N et al (2002) Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13:470–479
Pecoits-Filho R, Araujo MR, Lindholm B et al (2002) Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant 17:1480–1486
Goodlad C, Brown AE (2011) Encapsulating peritoneal sclerosis: what have we learned? Semin Nephrol 31:183–198
Io H, Hamada C, Ro Y, Ito Y, Hirahara I, Tomino Y (2004) Morphologic changes of peritoneum and expression of VEGF in encapsulated peritoneal sclerosis rat models. Kidney Int 65(5):1927–1936
Lafrannce JP, Lẽtourneau I, Ouimet D et al (2008) Successful treatment of encapsulating peritoneal sclerosis with immunosuppressive therapy. Am J Kidney Dis 51:e7–e10
Kawanishi H, Watanabe H, Moriishi M, Tsuchiya S (2005) Successful surgical management of encapsulating peritoneal sclerosis. Perit Dial Int 25(4):S39–S47
Del Peso G, Bajo MA, Gil F, Aguilera A et al (2003) Clinical experience with tamoxifen in peritoneal fibrosing syndromes. Adv Perit Dial 19:32–35
Sarioglu S, Sis B, Celik A et al (2006) Quantitative digital histochemistry with methenamine silver staining in renal allograft biopsies excluding pure chronic allograft nephropathy cases. Transplant Proc 38:490–491
Gotloib L, Wajsbrot V, Shostak A (1995) Morphology of the peritoneum: effect of peritoneal dialysis. Perit Dial Int 15:9–11
Mandl-Weber S, Cohen CD, Haslinger B, Kretzier M, Sitter T (2002) Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int 61:570–578
Stavenuiter AWD, Schilte MN, Ter Wee PM, Beelen RHJ (2011) Angiogenesis in peritoneal dialysis. Kidney Blood Press Res 34:245–252
Kawanishi H, Kawaguchi Y, Fukui H et al (2004) Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 44:729–737
Di Lauro R, De Ruggiero P, di Lauro R, di Lauro MT, Romano MR (2010) Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol 248(6):785–791
Oneill EC, Qin Q, Van Bergen NJ et al (2010) Antifibrotic activity of bevacizumab on human Tenon’s fibroblasts in vitro. Invest Ophthalmol Vis Sci 51(S1):6524–6532
Huang Y, Feng H, Kan T et al (2013) Bevacizumab attenuates hepatic fibrosis in rats by inhibiting activation of hepatic stellate cells. PLoS ONE 8(8):e73492
Ignjatovic D, Aasland K, Pettersen M et al (2010) Intra-abdominal administration of bevacizumab diminishes intra-peritoneal adhesions. Am J Surg 200(2):270–275
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Ada, S., Ersan, S., Sifil, A. et al. Effect of bevacizumab, a vascular endothelial growth factor inhibitor, on a rat model of peritoneal sclerosis. Int Urol Nephrol 47, 2047–2051 (2015). https://doi.org/10.1007/s11255-015-1116-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1116-8