Abstract
Objectives
The objective of this study was to evaluate the significance of docetaxel-based chemotherapy in elderly Japanese men with metastatic castration-resistant prostate cancer (CRPC).
Materials and methods
This study included a total of 159 consecutive patients aged ≥75 years with mCRPC who were treated with docetaxel-based chemotherapy. The efficacy and tolerability of this therapy were retrospectively analyzed.
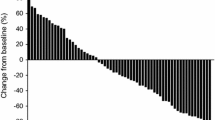
Results
In these 159 patients, the median age and prostate-specific antigen (PSA) level before docetaxel-based chemotherapy were 78 years and 44.0 ng/ml, respectively. Of these patients, 42 (26.4 %) and 117 (73.6 %) received docetaxel as a weekly (30 mg/m2) and 3-weekly (70 mg/m2) regimen, respectively, and estramustine was administered combining with docetaxel in 77 (48.4 %). Following docetaxel-based chemotherapy, PSA declined in 118 patients (74.3 %), including 87 (54.6 %) achieving a PSA decline ≥50 %, and the median progression-free survival and overall survival (OS) were 2.9 and 23.2 months, respectively. Of several factors examined, univariate analysis identified performance status (PS), significant clinical pain, bone metastasis, schedule of treatment, treatment cycle, and PSA response as significant predictors of OS, of which only PS, treatment cycle, and PSA response appeared to be independently associated with OS on multivariate analysis. The major grade 3–4 toxicities were myelosuppression, including neutropenia, anemia, and thrombocytopenia in 78 (49.1 %), 22 (13.8 %), and 14 (8.8 %), respectively.
Conclusions
These findings suggest that docetaxel-based chemotherapy is clinically feasible in Japanese men aged ≥75 years with mCRPC considering the cancer control as well as safety associated with this therapy.



Similar content being viewed by others
References
Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T (2011) Cancer incidence and incidence rates in Japan in 2005: based on data from 12 population-based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 41:139–147
Cancer Registration Committee of the Japanese Urological Association (2005) Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol 12:46–61
Donkena KV, Yuan H, Young CY (2010) Recent advances in understanding hormonal therapy resistant prostate cancer. Curr Cancer Drug Targets 10:402–410
Beltran H, Beer TM, Carducci MA et al (2011) New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol 60:279–290
Droz JP, Balducci L, Bolla M et al (2010) Management of prostate cancer in older men: recommendations of a working group of the International Society of Geriatric Oncology. BJU Int 106:462–469
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Ide H, Kikuchi E, Kono H et al (2010) Docetaxel in combination with prednisolone for hormone refractory prostate cancer. Jpn J Clin Oncol 40:79–84
Matsumoto A, Inoue A, Yokoi S et al (2009) Evaluation of docetaxel plus estramustine in the treatment of patients with hormone-refractory prostate cancer. Int J Urol 16:687–691
Naito S, Tsukamoto T, Koga H et al (2008) Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter phase II trial in Japan. Jpn J Clin Oncol 38:365–372
Takaha N, Okihara K, Kamoi K et al (2011) Feasibility of tri-weekly docetaxel-based chemotherapy for elderly patients (age 75 and older) with castration-resistant prostate cancer. Urol Int 87:263–269
Italiano A, Ortholan C, Oudard S et al (2009) Docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-resistant prostate cancer. Eur Urol 55:1368–1375
Takechi H, Ito K, Yamamoto T, Miyakubo M, Ohi M, Suzuki K (2008) Prostate-specific antigen kinetics in screen-detected prostate cancer in Japan. Urology 72:1111–1115
Maekawa K, Harakawa N, Yoshimura T et al (2010) CYP3A4*16 and CYP3A4*18 alleles found in East Asians exhibit differential catalytic activities for seven CYP3A4 substrate drugs. Drug Metab Dispos 38:2100–2104
Misumi T, Yamamoto Y, Murakami T et al (2010) Genetic alterations at 13q14 may correlate with differences in the biological behavior of prostate cancer between Japanese and Caucasian men. Urol Int 84:461–466
Phan VH, Moore MM, McLachlan AJ, Piquette-Miller M, Xu H, Clarke SJ (2009) Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol 5:243–257
Miyake H, Sakai I, Terakawa T, Harada KI, Fujisawa M. Oncological outcome of docetaxel-based chemotherapy for Japanese men with metastatic castration-resistant prostate cancer. Urol Oncol (in press)
Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M (2007) A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res 13:6396–6403
Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, Halabi S (2010) The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer 46:517–525
ten Tije AJ, Verweij J, Carducci MA et al (2005) Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. J Clin Oncol 23:1070–1077
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyake, H., Sakai, I., Harada, Ki. et al. Significance of docetaxel-based chemotherapy as treatment for metastatic castration-resistant prostate cancer in Japanese men over 75 years old. Int Urol Nephrol 44, 1697–1703 (2012). https://doi.org/10.1007/s11255-012-0223-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-012-0223-z