Abstract
Introduction
Hemojuvelin (HJV) is highly expressed in the liver, skeletal muscles, and heart, seems to play a role in iron absorption and release from cells, and has anti-inflammatory properties. Moreover, HJV plays an essential role in the regulation of hepcidin expression, specifically in the iron-sensing pathway. Hepcidin has emerged as a key regulator of iron homeostasis. In this study we tested for the first time the hypothesis that HJV is related to iron metabolism in hemodialysis (HD) patients.
Methods
Iron status, complete blood count, and serum creatinine, albumin, and lipids were assessed, using standard laboratory methods. Serum levels of soluble transferrin receptor (sTFR), high-sensitivity CRP, IL-6, hepcidin, and HJV were measured using commercially available kits.
Results
Serum HJV, hepcidin, ferritin, IL-6, hsCRP, and serum creatinine were significantly higher (all P < 0.001), whereas serum iron, sTFR, transferrin, hemoglobin, and erythrocyte count were significantly lower in HD patients, compared to healthy volunteers (all P < 0.001). In univariate analysis, HJV was strongly correlated (P < 0.001) with ferritin, transferrin saturation, and TIBC, as well as with hsCRP, hepcidin, Kt/V (P < 0.01) and residual renal function, the presence of diabetes, APKD, and coronary heart disease. Predictors of HJV level in multiple regression analysis were ferritin (beta value was 0.50, P = 0.00004) and transferrin saturation (beta value was 0.47, P = 0.0002), explaining 81% of the HJV variations.
Conclusions
Serum HJV is elevated in HD patients and related predominantly to kidney function and iron metabolism. However, HJV is probably not correlated to inflammation. HJV appears to be a new player in iron metabolism in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemojuvelin (HJV) is a membrane protein that is responsible for an iron overload condition known as juvenile hemochromatosis [1]. HJV is highly expressed in the liver, skeletal muscles, and heart [2]; it seems to play a role in iron absorption and release from cells and also has anti-inflammatory properties [3]. One feature of the pathogenesis of juvenile hemochromatosis is that patients have low to undetectable urinary hepcidin levels, suggesting that HJV is a positive regulator of hepcidin, the central iron regulatory hormone. As a result, low hepcidin levels result in increased intestinal iron absorption. Moreover, HJV plays an essential role in the regulation of hepcidin expression, specifically in the iron-sensing pathway [4]. Hepcidin has recently emerged as a key regulator of iron homeostasis [5]. Hepcidin is a small defensin-like peptide produced by the hepatocytes. Its production is modified in response to anemia, hypoxia, and inflammation [6]. Stimulation by IL-6 is necessary for hepcidin synthesis during inflammation (“the IL-6-hepcidin axis”), but not for hepcidin activation during iron overload [7]. According to Niederkofler et al. [4], dietary iron-sensing and inflammatory pathways converge in the regulation of hepcidin, but how these two pathways intersect remains unclear. Hepcidin was found to be elevated in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) undergoing renal replacement therapy [8, 9]. Moreover, in the first studies, relations between prohepcidin and anemia were described [10, 11]. There are no data available on HJV levels in CKD; however, the mean soluble HJV concentration in patients with anemia of chronic disease was reported to be significantly higher than in controls [12].
This study was performed to test for the first time the hypothesis that HJV is related to iron metabolism in HD patients and also to assess the correlation between HJV and hepcidin and other markers of inflammation.
Patients and methods
Study population
The study included 110 prevalent patients on maintenance HD. Inclusion criteria were: a stable clinical state, no thrombosis or inflammation (serum CRP below 6 mg/l using a semiquantitative method), and absence of acute cardiovascular complications (including uncontrolled hypertension, acute coronary syndrome, and acute heart failure). Patients with renal graft failure and/or on immunosuppressive therapy were excluded. The control group consisted of 30 healthy volunteers. All patients were fully informed about the study and gave their consent. The study was approved by the local Medical University Ethics Committee.
Laboratory measurements
Blood was drawn in all patients in the morning between 8.00 am. and 9.00 am. before the onset of the midweek dialysis session and before heparin administration. Blood for urea and creatinine measurements for Kt/V calculation was taken after hemodialysis from the arterial line of the hemodialysis system, immediately before discontinuation of the extracorporeal circulation. Enoxaparin (Clexane, Aventis) was used as an anticoagulant during hemodialysis and was given as a single intravenous injection at the beginning of each dialysis session. The blood drawn from the patients was centrifuged at 2500g for 15 min at room temperature to serum. Samples were aliquotted and stored at −40°C before assays. High-sensitivity CRP was studied using kits from American Diagnostica, Greenwich, CT, USA. Soluble transferring receptor (sTfR) and interleukin-6 (high sensitivity) and TNFα (high sensitivity) were studied using kits from R&D (Abington, UK). Hepcidin was measured by an assay from Bachem, UK. HJV was assessed using commercially available kit from uscnb.life, China. Hemoglobin, total protein, serum lipids, albumin, CRP (for screening purposes, using a semiquantitative method in which values below 6 mg/l are not calculated and are considered normal), serum iron, TIBC, ferritin, pH, and bicarbonate were measured by standard laboratory methods in a central laboratory.
Statistical analysis
The data were analyzed using Statistica 8.0. computer software. If possible, data were logarithmically transformed to achieve a normal distribution. Normality of variable distribution was tested using the Shapiro–Wilk W-test. Measurements normally distributed are reported as means ± SD, and non-normally distributed data are expressed as a median and minimal-maximal value. Analysis of variance (ANOVA) (with post hoc Tukey test for unequal groups) or Kruskall–Wallis ANOVA (the difference between the mean of two variables was calculated by Mann–Whitney U-test) was used in statistical analysis to compare differences between groups with P < 0.05 considered statistically significant, when appropriate. Linear regression analysis employed Pearson or Spearman coefficients as appropriate. Multiple regression analysis was used to determine independent factors affecting the dependent variable. Factors showing linear correlation with HJV (P < 0.1) were included in the analysis.
Results
Patients and dialysis characteristics
The study included 110 stable patients on maintenance HD (mean age was 58.64 ± 14.64 years). Median time on HD was 38 months (range 3–278 months). All the patients were treated with regular HD for 4–5 h three times a week. Blood flow was 180–280 ml/min (median 250 ml/min) and dialysate flow was 500 ml/min. Ultrafiltration varied according to the patient’s actual weight. All patients were dialysed using low-flux polysulphone membranes (Fresenius, Bad Homburg, Germany) and low-flux modified cellulose membranes (Terumo, Althin, Gambro, Braun, Nipro) with bicarbonate-buffered dialysate.
The causes of ESRD included chronic glomerulonephritis (n = 34), diabetic nephropathy (n = 32), polycystic kidney disease (ADPKD) (n = 12), hypertensive nephropathy (n = 12), and others or unknown (n = 20). One hundred patients were treated with erythropoiesis-stimulating agents and 101 with anti-hypertensive drugs.
Hemojuvelin in HD patients
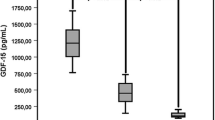
Serum HJV, hepcidin, ferritin, IL-6, and hsCRP were significantly higher in HD (all P < 0.001), whereas serum iron, sTFR, transferrin, and hemoglobin were significantly lower in HD patients, compared to healthy volunteers (Table 1). In univariate analysis, HJV was strongly correlated with ferritin (r = 0.57, P < 0.001) (Fig. 1), transferrin saturation (r = 0.40, P < 0.001), and TIBC (−r = 0.35, P < 0.001) (Fig. 2). HJV was also correlated with pH (r = −0.27, P < 0.01), IL-6 (r = 0.25, P < 0.001), the presence of diabetes (r = 0.26, P < 0.01), Kt/V (r = 0.28, P < 0.01), and hepcidin (r = −0.31, P < 0.01) and weakly correlated with TNF alpha (r = −0.21, P < 0.05), APKD (r = 0.23, P < 0.05), hsCRP (r = −0.22, P < 0.05) (Fig. 3), the presence of CAD (r = −0.25, P < 0.05), prohepcidin (r = −0.22, P < 0.05), and ESA dose (r = 0.19, P < 0.05). HJV was significantly higher in diabetic HD patients when compared to non-diabetic HD patients (6.90 ± 3.29 mg/l vs. 3.72 ± 0.33 mg/l, P < 0.01) and also in patients with ADPKD compared to other causes of ESRD, except diabetic nephropathy (4.36 ± 2.76 mg/l vs. 1.12 ± 1.01 mg/l, P < 0.05). HJV was significantly higher in patients without residual renal function when compared to patients with residual renal function (4.69 ± 2.29 mg/l vs. 3.18 ± 2.54 mg/l, P < 0.05). In patients with Hb over 12 g/dl, HJV was not significantly different in those with Hb lower than 12 g/dl. We also assessed the effect of a single HD session on HJV levels (n = 37, without diabetes) and found that HJV was significantly lower after hemodialysis when compared to pre-dialysis values (3.40 ± 1.08 mg/l vs. 2.78 ± 0.79 mg/l, P < 0.05). We detected HJV in the urine of the healthy volunteers and HD patients, and in the ultrafiltrate of HD patients. Predictors of HJV in multiple regression analysis were ferritin (beta value was 0.22, P = 0.045), TIBC (beta value was −0.52, P = 0.0012), and the presence of ADPKD (beta value was 0.59, P = 0.000026), explaining 72% of the HJV variations.
Discussion
In the present study, we describe for the first time hemojuvelin quantification in human serum of chronic HD patients, by using a commercially available laboratory kit. In the study of Brasse-Lagnel et al. [12], who developed an assay for HJV determination, they found that in healthy controls, serum HJV values ranged from 0.88 to 1.14 mg/l, with no difference between men and women. They also reported that, in a group of patients with anemia of chronic disease, serum HJV concentrations were significantly higher than in controls. The soluble form of HJV is thought to be a negative regulator of hepcidin expression, but its role in the regulation of hepcidin following an inflammatory stimulus remains unclear. In our study, we found an elevated HJV level in HD patients compared to healthy volunteers. In addition, we found that patients with diabetes and patients with ADPKD had higher HJV levels than patients with other causes of ESRD.
In our study, we found that patients with residual renal function had lower HJV levels; however, in the multivariate analysis, presence or absence of residual renal function was not related to HJV. An HD session was responsible for a decrease in HJV concentration. In our HD population, HJV was independently related to ferritin, TIBC, and the presence of ADPKD. In a recent study published by Verdalles et al. [13] in HD patients not treated with ESA, the presence of ADPKD and hepatitis C virus (HCV) infection were independent predictors for the absence of anemia in HD, probably due to the endogenous erythropoietin production. However, in our study, the presence of HBV or anti-HCV antibodies did not influence HJV levels.
The possible explanation of the effect of ADPKD on HJV might be associated with the erythropoietin production by the renal cells. As reported by Eckardt et al. [14], single interstitial cells juxtaposed to proximal tubular cysts may produce EPO independently of the oxygen pressure inside the cysts, which ameliorates the anemia in ESRD. They suggested that the stimulus for EPO production was related to the alteration in the functional architecture of peritubular tissue. In type 2 diabetes, ferritin was reported to be associated with insulin resistance [15]. As suggested by Jiang et al. [16], elevated hepcidin might have adaptive value through down-regulated iron absorption and play an important role in the pathogenesis of type 2 diabetes. On the other hand, beta-cells iron deposits-induced decreased secretory capacity might be of primary importance to trigger diabetes in HFE patient [17]. Up to date, there are no data available on HJV and diabetes.
In an animal model, HJV expression was repressed in mice at the transcriptional level, by pro-inflammatory stimulus [18, 19]. We found that HJV was independently related only to ferritin, but not to hsCRP or other inflammatory cytokines. In addition, Babitt et al. [20] showed that injections of high doses of recombinant HJV revert the induction of hepcidin mRNA by interleukin-6 [5]. Brasse-Lagnel et al. [12] found in their study an inverse correlation between measured serum hemojuvelin and circulating iron concentrations and with C-reactive protein, an index of inflammation. They suggested that the inverse correlation between soluble hemojuvelin and serum iron was in accordance with the effect observed in vitro, in which treatment of cells with iron resulted in decreased release of soluble hemojuvelin [2, 21]. They also reported a correlation between soluble hemojuvelin and ferritin (r = 0.62; P < 0.0001) in a whole group of subjects with anemia of chronic disease and in healthy volunteers [10]. We found in univariate analysis that HJV was related with ferritin in HD patients (r = 0.57, P < 0.001). In addition, we perform a multivariate analysis and found that ferritin was also a predictor of HJV in this population. However, Brasse-Lagnel et al. [12] did not observe any correlation between hepcidin and hemojuvelin levels in the serum of patients with anemia of chronic disease. We also did not find a correlation between hepcidin and HJV in our HD population. Nevertheless, they suggested that the nature of hemojuvelin inducers (inflammatory conditions or iron deficiency), as well as the source tissue, remained to be demonstrated [10]. They also indicated that circulating hemojuvelin could be a potential new treatment for anemia associated with excess hepcidin, as in patients with iron-refractory iron-deficient anemia, with mutations in the TMPRSS6 gene or in inflammatory anemia. In the recent study by Theurl et al. [21], a significantly reduced mHJV expression was observed in rats with anemia of chronic disease (ACD) on an iron-deficient diet as compared to ACD rats. They suggested different signaling pathways and hierarchies between inflammatory anemia (ACD) and inflammatory anemia with associated true iron deficiency (ACD/IDA). The role of hepcidin in renal anemia [22] as well as the regulation of hepcidin via iron’s induction of hepcidin gene transcription via BMP-6/Smad signaling pathway including HJV, transferrin receptor 2, and other proteins was elegantly presented by Coyne et al. [23].
Since hepcidin expression is positively regulated by body iron load, the potential role of sHJV in systemic iron homeostasis was recently investigated. In transfected cell lines, sHJV release was negatively regulated by either iron-saturated Tf or non-Tf iron [1, 2, 24]. Moreover, in animal model of acute iron deficiency, decreased serum Tf saturation was associated with the marked suppression of hepcidin expression and a significant increase in serum HJV [24]. Therefore, these findings are suggestive of the important role of sHJV in the regulation of hepcidin expression in response to body iron status. However, the underlying mechanism for iron-regulated HJV release has not been fully elucidated. In animal studies, HJV mRNA expression in either skeletal muscle or liver was not regulated by iron [18, 24]. Silvestri et al. [25] showed that in hepatoma cell line the expression of furin was regulated by hypoxia and predicted that HJV release was likely regulated by the levels of furin. However, Krijt et al. [26] did not detect any change of furin expression in either skeletal muscle or liver from mice with hypoxia. On the other hand, because furin is ubiquitously expressed in all tissues, including skeletal muscle and hepatocytes where HJV is expressed. The recent study by Theurl et al. [27], using the well-established rat model of ACD, provided novel evidence for effective treatment of ACD by blocking endogenous hepcidin production using the small molecule dorsomorphin derivative LDN-193189 or the protein soluble hemojuvelin-Fc (HJV.Fc) to inhibit bone morphogenetic protein-Smad-mediated signaling required for effective hepcidin transcription. This pharmacological inhibition of hepcidin expression results in mobilization of iron from the reticuloendothelial system, stimulation of erythropoiesis, and correction of anemia. They concluded that hepcidin-lowering agents are a promising new class of pharmacologic drugs to effectively combat anemia of chronic diseases.
Emerging evidence supports that serum HJV plays a potentially important role in iron homeostasis. However, it remains to be determined how HJV release is regulated by iron. In HD patients, iron metabolism is disturbed and there are many factors involved in its regulation. We found that HJV was elevated in HD patients and correlated to kidney function and iron status, but not to inflammation or to presence of anemia. It appears that HJV could be a new player in the iron metabolism in HD patients; however, the precise mechanism is yet to be elucidated in further studies.
References
Silvestri L, Pagani A, Fazi C, Gerardi G, Levi S, Arosio P, Camaschella C (2007) Defective targeting of hemojuvelin to plasma membrane is a common pathogenetic mechanism in juvenile hemochromatosis. Blood 109:4503–4510
Lin L, Goldberg YP, Ganz T (2005) Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood 106:2884–2889
Rodriguez Martinez A, Niemela O, Parkkila S (2004) Hepatic and extrahepatic expression of the new iron regulatory protein hemojuvelin. Haematologica 89:1441–1445
Niederkofler V, Salie R, Arber S (2005) Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest 115:2180–2186
Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O (2001) A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276:7811–7819
Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S (2001) Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA 98:8780–8785
Ganz T (2003) Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102:783–788
Malyszko J, Malyszko JS, Kozminski P, Mysliwiec M (2009) Type of renal replacement therapy and residual renal function may affect prohepcidin and hepcidin. Ren Fail 31:876–883
Malyszko J, Malyszko JS, Mysliwiec M (2009) A possible role of hepcidin in the pathogenesis of anemia among kidney allograft recipients. Transplant Proc 41:3056–3059
Małyszko J, Małyszko JS, Hryszko T, Pawlak K, Mysliwiec M (2005) Is hepcidin a link between anemia, inflammation and liver function in hemodialyzed patients? Am J Nephrol 25:586–590
Eleftheriadis T, Kartsios C, Liakopoulos V, Antoniadi G, Ditsa M, Papadopoulos C, Anifandis G, Skirta A, Markala D, Stefanidis I (2006) Does hepcidin affect erythropoiesis in hemodialysis patients? Acta Haematol 116:238–244
Brasse-Lagnel C, Poli M, Lesueur C, Grandchamp B, Lavoinne A, Beaumont C, Bekri S (2010) Immunoassay for human serum hemojuvelin. Haematologica 95:2031–2037
Verdalles U, Abad S, Vega A, Ruiz Caro C, Ampuero J, Jofre R, Lopez-Gomez JM (2011) Factors related to the absence of anemia in hemodialysis patients. Blood Purif 32:69–74
Eckardt KU, Möllmann M, Neumann R, Brunkhorst R, Burger HU, Lonnemann G, Scholz H, Keusch G, Buchholz B, Frei U (1989) Erythropoietin in polycystic kidneys. J Clin Invest 84:1160–1166
Aso Y, Takebayashi K, Wakabayashi S, Momobayashi A, Sugawara N, Terasawa T, Naruse R, Hara K, Suetsugu M, Morita K, Inukai T (2010) Relation between serum high molecular weight adiponectin and serum ferritin or prohepcidin in patients with type 2 diabetes. Diabetes Res Clin Pract 90:250–255
Jiang F, Sun ZZ, Tang YT, Xu C, Jiao XY (2011) Hepcidin expression and iron parameters change in Type 2 diabetic patients. Diabetes Res Clin Pract 93:43–48
Ramey G, Faye A, Durel B, Viollet B, Vaulont S (2007) Iron overload in Hepc1(-/-) mice is not impairing glucose homeostasis. FEBS Lett 581:1053–1057
Krijt J, Vokurka M, Chang KT, Necas E (2004) Expression of Rgmc, the murine ortholog of hemojuvelin gene, is modulated by development and inflammation, but not by iron status or erythropoietin. Blood 104:4308–4310
Constante M, Wang D, Raymond VA, Bilodeau M, Santos MM (2007) Repression of repulsive guidance molecule C during inflammation is independent of Hfe and involves tumor necrosis factor-alpha. Am J Pathol 170:497–504
Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY (2007) Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest 117:1933–1939
Theurl I, Schroll A, Nairz M, Seifert M, Theurl M, Sonnweber T, Kulaksiz H, Weiss G (2011) Pathways for the regulation of hepcidin expression in anemia of chronic disease and iron deficiency anemia in vivo. Haematologica. doi:10.3324/haematol.2011.048926
Eleftheriadis T, Liakopoulos V, Antoniadi G, Kartsios C, Stefanidis I (2009) The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin Dial 22:70–77
Coyne DW (2011) Hepcidin: clinical utility as a diagnostic tool and therapeutic target. Kidney Int 80:240–244
Zhang AS, Anderson SA, Meyers KR, Hernandez C, Eisenstein RS, Enns CA (2007) Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J Biol Chem 282:12547–12556
Silvestri L, Pagani A, Camaschella C (2008) Furin mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood 111:924–931
Krijt J, Fujikura Y, Sefc L, Vokurka M, Hlobenova T, Necas E (2009) Hepcidin downregulation by repeated bleeding is not mediated by soluble hemojuvelin. Physiol Res 59:53–59
Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, Wolf D, Seifert M, Sun CC, Babitt JL, Hong CC, Menhall T, Gearing P, Lin HY, Weiss G (2011) Pharmacologic inhibition of hepcidin expression reverses anemia of chronic disease in rats. Blood 118:4977–4984
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Malyszko, J., Malyszko, J.S., Levin-Iaina, N. et al. Is hemojuvelin a possible new player in iron metabolism in hemodialysis patients?. Int Urol Nephrol 44, 1805–1811 (2012). https://doi.org/10.1007/s11255-011-0084-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-011-0084-x