Abstract
Soot is known to cause high wear in engine components and this is a problem with engine oils between extended drain intervals. Although several wear mechanisms have been suggested, exact soot-induced wear mechanisms are still not fully understood. This study aims to investigate the additive adsorption mechanism (specifically anti-wear additive) on carbon black (CB) particles (as a soot surrogate). This mechanism is one of the main mechanisms responsible for high wear. Base oil (BO) was used to exclude the effect of other additives that are present in fully formulated oil. Zinc dialkyldithiophosphate (ZDDP) anti-wear additive was added to the BO and then the mixture, which will be referred to as the model oil, was aged with CB particles. The resulting oils were then chemically characterised by using ICP and FTIR analysis techniques. Finally, the CB particles were filtered out and analysed using EDX. The results showed that both zinc and phosphorous which are chemical elements from ZDDP anti-wear additive were adsorbed on CB particles in the oil phase. This additive adsorption mechanism can lead to increased wear in the engine components due to lack of additives in the oil. This study highlights the importance of understanding the additive adsorption on soot particles since this mechanism will reduce the engine oil life and increase frequency of oil changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soot is known to induce high wear in engine components. The effect of soot contamination in engine oils on wear of engine components has been investigated in many studies [1,2,3,4,5,6]. Several mechanisms such as abrasion [4, 7,8,9], oil starvation [10,11,12], competitive adsorption [13,14,15], additive adsorption on soot [2, 5, 11, 16] and corrosive–abrasive wear [4, 6, 17, 18] have been suggested by which soot induces high wear.
The parasitic consumption of zinc dialkyldithiophosphate (ZDDP) on soot particles in diesel engine oils is known to occur but the extent of it is not fully understood. This effect of soot is in addition to its abrasion characteristics [19] and recently proposed corrosive–abrasive mechanism [6, 17]. Soot has a tremendous surface activity, so it entraps the effective ZDDP decomposition products and results in severe wear [20].
In 1981, Rounds [2] proposed an adsoprtion theory in which soot particles adsorb the decomposition products of anti-wear additives. Therefore, the concentration of the additives in the oil reduces, leading to high wear [2, 11]. Consequently, less anti-wear additives adsorb at the contact interface to form anti-wear films. This reduces the oil’s ability to protect the surfaces because prior to the formation of a tribofilm, soot adsorbs the anti-wear additives.
Hosonuma et al. [16] also showed that soot preferentially adsorbs zinc-containing compounds and very low amount of phosphorous compounds. They agreed with the Rounds theory of additive adsorption (particularly zinc) on soot. It was shown [16] that zinc concentration decreased dramatically from 930 to 20 ppm in BO + 1 wt% ZDDP in the presence of 2 wt% soot, while there was a little change in phosphorus and almost no change in sulphur concentration [16]. They also reported [16] a 48% increase in the zinc content of soot particles. However, they believed that the oils could still perform with the remaining phosphorus compounds within the oils. Gautam [21] also agreed with Rounds’ adsorption theory. However, Berbezier [14] stated that the adsorption of ZDDP additives on CB particles does not seem to be an important factor contributing to high wear. They showed [14] that none of the ZDDP elements can be detected on the CB particles. They believed that the adsoprtion strength is very weak.
From the literature, it can be concluded that there is no consensus on the adsorption of anti-wear additive, i.e. ZDDP, on carbon black (CB) particles. This research aims to focus on the adsoprtion of additives on CB particles in the oil phase. It also aims to quantify the amount of additives adsorbed on the CB particles at various CB levels and different oil ageing times. This study for the first time highlights the link between the amount of CB in the oil and additive adsorption.
2 Experimental Methodology
2.1 Materials and Analytical Techniques
2.1.1 Base oil and Anti-wear Additive
A base oil (BO), hereafter referred to as BO, was used to exclude the effect of other additives that are present in fully formulated oils. The BO tested was a Group III mineral oil. A secondary ZDDP anti-wear additive was also used in this study in order to create a series of model oils. The additives and the mineral BO were sourced from Total Raffinage, Solaize, France.
Table 1 shows the different levels of ZDDP concentration in the BO used for the experiments in this study. A hot plate and a stirrer were used to mix the ZDDP with the BO at 60 °C for 30 min. Some of the ZDDP concentrations used in this study are higher than the standard ZDDP concentrations in fully formulated oil (FFO). However, these oils were only used for calibration purposes in this study.
2.1.2 Oil Analysis
In this study, Fourier Transform InfraRed spectroscopy (FTIR) was used to identify the chemical composition in bulk oils during the ageing process and adsorption test. A Perkin Elmer FT-IR spectrometer within the range of 650–4000 cm−1 was used and the resolution of collected spectra was 4 cm−1.
Figure 1 shows the FTIR spectra of the BO and ZDDP used in this study. These three main peaks, which are from the base stock hydrocarbons, were present in both BO and ZDDP. IR bands between 2850 and 3000 and 1385–1500 cm−1 are attributed to alkyl C–H stretching and C–H bending, respectively. The peak at 722 cm−1 is also attributed to alkyl chains [22]. The peak region from 950 to 1020 cm−1 in ZDDP is the most relevant to this study. Within this region, strong bands associated with P–O–(C) vibrations in ZDDP are adsorbed [23]. This peak is the main difference between the BO and ZDDP spectra.
Figure 2 shows FTIR spectra of the model oils containing different concentrations of ZDDP in the region of 950–1020 cm−1. It can be clearly seen that the P–O–(C) peak appears by adding ZDDP to the BO. The intensity of this peak increased as more ZDDP concentration added to the BO. The areas of these peaks were also calculated to quantify the change in intensity by adding higher ZDDP concentration in BO.
Figure 3 shows the peak area in the region of 950–1020 cm−1 against different ZDDP concentrations in BO. It can be seen that there is an increase in the peak area by adding higher ZDDP concentration. This is a linear relationship between adding ZDDP and increase in the intensity of P–O–(C). Thus, FTIR is a reliable method to determine the presence of ZDDP in oil. It is also worth noting that it was difficult to calculate the area of P–O–(C) peak for low concentration of ZDDP (0.5 wt%) (Fig. 2), as the difference is within the data variation of the FTIR technique.
2.1.3 Carbon black particles
A commercially available Monarch 120 carbon black (Cabot Corporation, Massachusetts, USA) was used to simulate engine soot in this study. Although CB is not the exact replicate of engine soot, it is an ideal surrogate for soot since it allows repeatable experiments to be carried out and reliable data to be obtained. The Monarch 120 has been previously used in other studies and showed consistent results [24]. The individual CB particles used in this study were approximately 50 nm in diameter. The rounded particles were agglomerates of smaller particles and were 100–200 nm in size [6]. The hardness of CB particles has been reported [25] to be comparable to engine metal hardness. Considering that the chemical nature of CB and soot is very similar, it has been assumed that adsorption behaviour of CB and soot will be similar too.
2.1.4 The Adsorption Test of ZDDP on CB Particles
In the current study, all experiments were conducted using 1 wt% ZDDP in BO which is a realistic ZDDP concentration in the oil. The CB adsorption test scheme is shown in Fig. 4. These adsorption experiments were conducted to investigate the adsorption of ZDDP decomposition products on CB particles. Two different CB levels, 1 and 5 wt%, were added to the oil using stirrer and hot plate for 30 min at 60 °C. Then, the oil samples were aged for 2 and 96 h. The oil ageing was conducted artificially in the lab in accordance to the method explained in the previous studies [5, 26, 27]. Centrifugation was used to separate CB particles from the model oil prior to ICP measurement. The aged oil samples were centrifuged at 12,000 rpm for 2 h to separate CB particles from the oil.
2.1.5 ZDDP Concentration in Oil
Inductively Coupled Plasma (ICP) is an instrumental technique capable of performing chemical analysis on given samples, to within parts per trillion accuracy. ICP was used in this study to measure the concentration of ZDDP additive in the oils after mixing/ageing with CB particles. It is worth mentioning that the oil samples were centrifuged prior ICP analysis to remove CB particles. All ICP analyses were conducted by Millers Oils, Brighouse, UK.
Figure 5 indicates the zinc concentration in the BO after blending process using ICP. The BO had no additives and therefore ICP analysis showed no Zn element in BO (marked as zero in the graph). Thus, all the zinc concentration came from the ZDDP additive. It can be seen that there was a linear relationship between the added ZDDP values in the BO and zinc concentration measured by ICP. 1 wt% ZDDP concentration in BO which is the focus of this study showed 640 ppm Zn.
Both ICP and FTIR analyses indicated that these methods are appropriate to measure the level of ZDDP in the oil and to confirm ZDDP decomposition during the ageing process.
2.1.6 Carbon Black Particle Analysis
Energy dispersive X-ray (EDX) spectrometer was used in this study to provide information about the chemical composition of CB particles after being exposed to ZDDP additive. CB particles were removed from oil using centrifugation and dried in the oven for 24 h. EDX was carried out on ten different spots to check the uniformity of the compositions.
3 Results and Discussion
3.1 Oil Degradation in Presence of CB
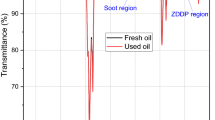
Figure 6 shows the images of aged oils after removing the CB particles using centrifuge. From Fig. 6, it can be seen that the oil samples are still clear and there is no sign of CB particles after 2 h of ageing and centrifuging. Therefore, FTIR analysis was conducted in order to check the presence of CB in oils after centrifugation. In FTIR analysis, the region around 2000 cm−1 is generally used [28] to determine the level of soot in the oil. The presence of soot in the oil causes a shift in the baseline of the spectrum because of absorption and scattering of light which can be clearly detected at 2000 cm−1. Figure 7 shows that there is no shift on the baseline around 2000 cm−1. Thus, these results confirm that all the CB particles were completely removed from the oil and the centrifugation was an appropriate method to separate the CB particles from the BO before ICP analysis.
FTIR confirmed that there is no CB left in the oil after centrifugation. Therefore, the dark colour observed on aged oils after 96 h shows that the oils experienced some level of degradation, suggesting the chemical modifications of the oil. Figure 8 shows that the oxidation peak at 1720 cm−1 appeared for 96-h aged oil samples, which indicates that the oil samples have gone through a degradation process. This change in colour was not seen after 2 h of ageing process. Further analyses such as FTIR and ICP were conducted on both oil samples to investigate additive decomposition during ageing process. Moreover, EDX analysis was conducted on CB particles to investigate additive adsorption on CB particles.
Figure 8 shows the FTIR spectra of aged oils (BO + 1 wt% ZDDP) in the presence of 1 and 5 wt% CB particles after 2 and 96 h of ageing process. As mentioned above, CB particles were removed from the oil using the centrifuge before FTIR analysis. Results showed that the P–O–(C) peak around 950–1020 cm−1 was still present after 2 h of ageing. However, this peak, P–O–(C), disappeared after 96 h of ageing regardless of CB concentration.
Figure 9 shows the area of P–O–(C) peak around 950–1020 cm−1 for oils containing 1 and 5 wt% CB after 2 h of ageing. In the presence of CB (both 1 and 5 wt%), the area of P–O–(C) peak reduced compared to the fresh oil. However, there is still sign of ZDDP in the oils suggesting that additive adsorption has partially occurred after 2 h of ageing. Moreover, higher concentration of CB in oil further decreased the peak area. This can be explained by higher numbers of CB particles and more surface area of particles to adsorb ZDDP additives.
As shown in Fig. 8, the P–O–(C) peak did not appear after 96 h of ageing, and thus the area of peak was not calculated for these samples. These results suggest that the ZDDP additives were mostly either adsorbed on CB particles or depleted after 96 h of ageing.
The aged oil samples were further analysed using ICP to investigate the zinc concentration from ZDDP additive after removing the CB particles. Table 2 shows the zinc concentration in the aged oil samples in the presence of 1 and 5 wt% CB particles. It can be seen that the fresh oil contained 640 ppm zinc. The aged oil with 1 wt% CB particles after 2 h of ageing showed slightly lower amount of zinc. However, adding more levels of CB particles, i.e. 5 wt%, reduced the zinc concentration significantly by approximately 55% after 2 h of ageing. This shows that additive adsorption on CB particles partially occurred at high levels of CB, 5 wt%, after 2 h of ageing. These results are in line other studies [16] showing that zinc content decreased appreciably with soot contamination.
After 96 h of ageing, no zinc remained in both samples regardless of CB concentration. These results indicate that as the ageing time increased more additives were adsorbed on the CB particles. FTIR results in Fig. 8 showed signs of oil degradation after 96 h of ageing (1720 cm−1); therefore, the reduction in zinc concentration can also be related to the additive depletion after 96 h of ageing.
To further investigate the additive adsorption on CB particles, EDX analysis was conducted on CB particles. The extracted CB particles from the aged oils were dried in an oven at 60 °C for 24 h to enable the EDX analysis to be undertaken. This technique was used to confirm the existence of ZDDP elements such as zinc and phosphate on CB particles after ageing. EDX data were also obtained from fresh (i.e. unused) CB Monarch 120 particles for comparison. Results of EDX spectrum showed that Monarch 120 particles were mainly composed of carbon (93 at.%), oxygen (6 at.%) and a very low level of sulphur (0.3 at.%). Table 3 shows the EDX spectra of CB particles removed from aged oils containing 1 and 5 wt% CB. From the results, it can be clearly seen that the CB particles from adsorption tests consist of zinc and phosphorous. However, there is no significant change in sulphur content of CB particles which is in line with previous studies [16]. Thus, it can be concluded that zinc and phosphorous from ZDDP additives adsorb on the CB particles. Hosonuma et al. [16] also showed that ZDDP decomposed quickly during the engine test and adsorbed on the soot particles. However, they showed [16] that mainly zinc, but very few phosphorous compound, adsorb on soot. Thus, they believed [16] that with the phosphorus compounds remaining in the oil, oil can still perform and protect the surfaces. However, the results from the current study provide evidence that high amount of phosphorous compound adsorb on the CB particles.
Moreover, as the ageing time increased from 2 to 96 h, higher concentration of zinc and phosphorous were observed on the CB particles. This is due to the thermal decomposition of ZDDP additives during the ageing process [29]. Zhang et al. [30] showed that the ageing process accelerated at high temperature and resulted in the higher formation of decomposition products. Ferguson et al. [29] also showed that thermal decomposition of ZDDP occurs at temperatures as low as 150 °C.
ZDDP decomposes to Zn and P compounds during the ageing process. As a result, these compounds interact with CB particles. EDX results confirmed that these compounds were adsorbed on CB particles in the oil phase during ageing the oil. These results are also in line with FTIR results which showed that the intensity of P–O–C peak reduced as ageing time increased.
The results from this study confirm that ZDDP decomposition compounds interact with CB particles. As a result of additive adsorption on CB particle, the amounts of additives in the oil phase reduce and affect the performance of oil. This study also showed that the higher concentration of CB led to more additive adsorption due to more and larger active surface area of CB particles. It is worth nothing that the model oil (BO + ZDDP) was used in this study in order to focus on the interaction between anti-wear additive and CB particles only. The authors have conducted another research [5] using FFO which showed that the anti-wear additive adsorption on CB particles occurred. However, soot can also adsorb other additives such as detergents and dispersants in the engine oil. Further study is required on the interaction between soot and other additives in FFO.
4 Conclusions
The adsorption of ZDDP decomposition products on CB particles was investigated in this study. CB particles are polar in nature and have a significant surface activity. The experimental results in this study demonstrated that the ZDDP additive adsorption on CB particles occurs. Previous studies [2, 16] only confirmed the adsorption of zinc on CB particles. However, in the current study it was experimentally shown that not only zinc but also phosphorous from ZDDP adsorbs on the CB particles in the oil phase. This mechanism will reduce oil’s ability to form anti-wear tribofilm and protect surfaces leading to high wear. This information will help oil manufacturer to consider the interaction between anti-wear additive and soot contamination in engine oil’s formulation in order to improve oil’s performance in the presence of CB.
It was also shown that additive adsorption of ZDDP on CB particles depends on test conditions such as CB concentration and ageing time of the oil. Higher concentration of CB increases the probability of the additive adsorption on CB particles. This can be related to the higher surface area of CB with higher levels of CB in oil which leads to more additive adsorption. Also, longer ageing process leads to higher additive adsorption on CB particles due to decomposition of ZDDP additive during longer ageing hours of the oil.
References
Green, D.A., Lewis, R.: Effect of soot on oil properties and wear of engine components. J. Phys. 40(18), 5488 (2007)
Rounds, F.G.: Soots from used diesel-engine oils: their effects on wear as measured in 4-ball wear tests. SAE International (1981)
Green, D.A., Lewis, R.: Investigation of soot contaminated lubricant wear mechanisms. SAE International (2007)
Torrance, M.: Wear of lubricated steel in the presence of dispersed carbon. In: IMechE Mission of Tribology Conference. (2004)
Motamen Salehi, F., Morina, A., Neville, A.: The effect of soot and diesel contamination on wear and friction of engine oil pump. Tribol. Int. 115(Supplement C), 285–296 (2017)
Motamen Salehi, F., et al.: Corrosive–abrasive wear induced by soot in boundary lubrication regime. Tribol. Lett. 63(2), 19 (2016)
Ryason, P.R., Chan, I.Y., Gilmore, J.T.: Polishing wear by soot. Wear 137(1), 15–24 (1990)
Kuo, C.C., et al.: Wear mechanism in cummins M-11 high soot diesel test engines. SAE International 981372 (1998)
Cadman, W., Johnson, J.H.: The study of the effect of exhaust gas recirculation on engine wear in a heavy-duty diesel engine using analytical ferrography. SAE International (1986)
Skurai, T., Yoshida, K.: Tribological behaviour of dispersed phase systems. In: International Tribology Conference (1987)
Green, D.A., Lewis, R.: The effects of soot-contaminated engine oil on wear and friction: a review. Automob. Eng. 222(9), 1669–1689 (2008)
Green, D.A., Lewis, R., Dwyer-Joyce, R.S.: Wear effects and mechanisms of soot-contaminated automotive lubricants. Eng. Tribol. 220, 159–169 (2006)
Rounds, F.G.: Carbon: cause of diesel engine wear? SAE International (1977)
Berbezier, I., Martin, M., Kapsa, Ph.: The role of carbon in lubricated mild wear. Tribol. Int. 19(3), 115–122 (1986)
Corso, S., Adamo, R.: The effect of diesel soot on reactivity of oil additives and valve train materials. SAE paper (1984)
Hosonuma, K., Yoshida, K., Matsunaga, A.: The decomposition products of zinc dialkyldithiophosphate in an engine and their interaction with diesel soot. Wear 103(4), 297–309 (1985)
Olomolehin, Y., Kapadia, R., Spikes, H.: Antagonistic interaction of antiwear additives and carbon black. Tribol. Lett. 37(1), 49–58 (2010)
Ratoi, M., et al.: The influence of soot and dispersant on ZDDP film thickness and friction. Lubr. Sci. 17, 25–43 (2004)
Nagai, I., et al.: Soot and valve train wear in passenger car diesel engines. SAE Technical Paper 831757. SAE International (1983)
Pawlak, Z.: Tribochemistry of Lubricating Oils, 1st edn. Elsevier, Amsterdam (2003)
Gautam, M., et al.: Effect of diesel soot contaminated oil on engine wear—investigation of novel oil formulations. Tribol. Int. 32(12), 687–699 (1999)
Lin-Vien, D., et al.: The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules. Academic Press, Boston (1991)
Tse, J.S., Song, Y., Liu, Z.: Effects of temperature and pressure on ZDDP. Tribol. Lett. 28(1), 45–49 (2007)
Devlin, M.T., et al.: Film formation properties of polymers in the presence of abrasive contaminants. SAE International 2002-01-2793 (2002)
Devlin, C.C., et al.: Biodiesel fuel effect on diesel engine lubrication. SAE International 2008-01-2375 (2008)
Charlotte, B., et al.: Investigation of long-term engine oil performance using lab-based artificial ageing illustrated by the impact of ethanol as fuel component. Tribol. Int. 46(1), 174–182 (2012)
CECL-48-A00: Oxidation stability of lubricating oils used in automotive transmissions by artificial ageing, Co-coordinating European Council for the Development of Performance Tests for Fuels, Lubricants and Other Fluids (2007)
Patty, D.J., Lokollo, R.R.: FTIR spectrum interpretation of lubricants with treatment of variation mileage. Adv. Phys. Theor. Appl. 52 (2016)
Ferguson, S., et al.: Analysis of ZDDP content and thermal decomposition in motor oils using NAA and NMR. Phys. Proc. 66, 439–444 (2015)
Zhang, X., et al.: Effect of temperature on the ageing behaviour of unsaturated ester-based lubricants. J. Synth. Lubr. 21(1), 1–11 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Motamen Salehi, F., Morina, A. & Neville, A. Zinc Dialkyldithiophosphate Additive Adsorption on Carbon Black Particles. Tribol Lett 66, 118 (2018). https://doi.org/10.1007/s11249-018-1070-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-018-1070-6