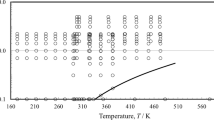
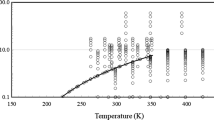
A new viscosity–temperature equation and corresponding chart have been developed to extend the range of the current ASTM viscosity–temperature charts. This new chart and equation extends the temperature and viscosity range for hydrocarbons and, for the first time, has the ability to extend to the low viscosity regime of halocarbons and low temperature fluids. The new equation and chart can linearize liquid viscosity data from 0.04 cSt and covers the temperature range from −210 to 500 °C for halocarbons and hydrocarbons. With a modification to the temperature scaling, the new equation also has the ability to fit liquid metal viscosity data. The new chart and equation cannot accurately linearize the viscosity with respect to temperature of fluids exhibiting strong molecular bonding (water, ammonia), fluids whose molecular structure consists of long coils (some long chained silicones), or fluid mixtures in which one fluid precipitates out of solution (wax precipitation).









Similar content being viewed by others
Notes
While Walther received credit for the equation correlating viscosity versus temperature, MacCoull’s earlier charts were based on the same form with an additive constant of 0.7. MacCoull’s work was clearly ahead of Walther’s, however he never disclosed the development of his chart until after the work of Walther had claimed recognition. This discrepancy was the one of the primary motivations of this intense historical review.
ASTM Standard D 341-43 implemented γ = 0.60 down to viscosities of 1.5 cSt, γ = 0.65 for the viscosity range 1.5–1.0 cSt, γ = 0.70 for 1.0–0.7 cSt, and γ = 0.75 for 0.7–0.4 cSt in the construction of the charts.
References
Reynolds O. (1886). Phil Trans Royal Soc London 177:157
ASTM 341-93 (1998). Standard viscosity–temperature charts for liquid petroleum products. ASTM International
Wright W.A. (1969). J Mater. JMLSA. 4(1):19
Vogel H. (1921). Physikalische Zeitschrift. 22: 645
G.S. Fulcher, J. Am. Ceramic Soc. 8(6) (1925) 339. Commemorative Reprint: J. Am. Ceramic Soc. 75(5) (1992) 1043
Walther C. (1928). Erdöl und Teer. 4: 510
Walther C. (1931). Erdöl und Teer. 7:382
Walther C. (1931). Maschinenbau. 10: 670
J.C. Geniesse and T.G. Delbridge, Proc. Second Mid-Year Meeting. Division of Refining, American Petroleum Institute (1932) 56–58
G. Barr, Proc. Gen. Discussion Lubrication Lubricants, Institution of Mechanical engineering 2 (1937) 217
Crouch R.F., Cameron A. (1961). J. Inst. Petroleum 47(453):307
C.J.A. Roelands, Correlational aspects of the viscosity–temperature–pressure relationship of lubricating oils, PhD Thesis, Delft University of Technology, Netherlands (1966)
Manning R.E. (1974). J. Testing Eval. 2(6):522
Scherer G.W. (1992). J. Am. Ceramic Soc. 75(5): 1060
Lubrication. A Technical Publication Devoted to the Selection and Use of Lubricants, A New Chart for Viscosity Temperature Relations. The Texas Company. 7(6) (1921) 5
International Critical Tables of Numerical Data, Physics, Chemistry and Technology, McGraw-Hill for The National Research Council. Vol. II, pp. 146–147 (1927)
Erk V.S., Eck H. (1936). Physikalische Zeitschrift. 37(4): 113
Murphy C.M., Romans J.B., Zisman W.A. (1951). Trans. Am. Soc. Mech. Eng. 71: 561
Lubrication. A Technical Publication Devoted to the Selection, Use of Lubricants. Viscosity—Effects of Temperature and Pressure. The Texas Company 36(6) (1950) 60
Stachowiak G.W., Batchelor A.W. (2005). Engineering Tribology, 3. New York, Elsevier, pp. 13–15
S. Blair, Proc. I MECH E, Part J. J. Eng. Tribolo. 218 (2004) 57
Vogel E., Küchenmeister C., Bich E., Laesecke A. (1998). J. Phys. Chem. Ref. Data 27(5): 947
http://webbook.nist.gov. NIST Chemistry Webbook. United States Department of Commerce. March 2006
Grosse A.V. (1966). J. Inorg. Nucl. Chem. 28: 31
Spells K.E. (1936). Proc. Phys. Soc. 48: 299
Tipton C.R., ed. (1960). Reactor Handbook; Volume I, Materials. 2. New York, Interscience Publishers, Inc. pp. 996-999
Acknowledgments
The Industrial Advisory Board of the Air Conditioning Research Center at the University of Illinois Urbana-Champaign provided funding for part of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seeton, C.J. Viscosity–temperature correlation for liquids. Tribol Lett 22, 67–78 (2006). https://doi.org/10.1007/s11249-006-9071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-006-9071-2