Abstract
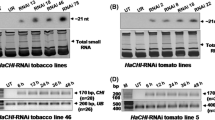
The success of Bt transgenics in controlling predation of crops has been tempered by sporadic emergence of resistance in targeted insect larvae. Such emerging threats have prompted the search for novel insecticidal molecules that are specific and could be expressed through plants. We have resorted to small RNA-based technology for an investigative search and focused our attention to an insect-specific miRNA that interferes with the insect molting process resulting in the death of the larvae. In this study, we report the designing of a vector that produces artificial microRNA (amiR), namely amiR-24, which targets the chitinase gene of Helicoverpa armigera. This vector was used as transgene in tobacco. Northern blot and real-time analysis revealed the high level expression of amiR-24 in transgenic tobacco plants. Larvae feeding on the transgenic plants ceased to molt further and eventually died. Our results demonstrate that transgenic tobacco plants can express amiR-24 insectice specific to H. armigera.


Similar content being viewed by others
References
Agrawal NB, Sachdev B et al (2013) Development associated profiling of chitinase and microRNA of Helicoverpa armigera identified Chitinase repressive microRNA. Sci Rep 3:2292
Ali I, Amin I et al (2013) Artificial microRNA-mediated resistance against the monopartite begomovirus Cotton leaf curl Burewala virus. Virol J 10(1):231
Ambros V (2004) The functions of animal microRNAs. Nature 431(7006):350–355
Asgari S (2012) MicroRNA functions in insects. Insect Biochem Mol Biol 43(4):388–397
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Behura SK (2007) Insect microRNAs: structure, function and evolution. Insect Biochem Mol Biol 37(1):3–9
Bischoff V, Vignal CC et al (2006) Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog 2(2):e14
Carrington JC, Ambros V (2003) Role of microRNAs in plant and animal development. Science 301(5631):336–338
Cortina C, Culianez-Macia FA (2004) Tomato transformation and transgenic plant production. Plant Cell Tissue Organ Cult 76(3):269–275
Daborn PJ, Lumb C et al (2012) Using Drosophila melanogaster to validate metabolism-based insecticide resistance from insect pests. Insect Biochem Mol Biol 42(12):918–924
Dowd PF, Lagrimini LM et al (1998) Differential leaf resistance to insects of transgenic sweetgum (Liquidambar styraciflua) expressing tobacco anionic peroxidase. Cell Mol Life Sci 54(7):712–720
Fire A, Xu S et al (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene jockeying” tool. Microbiol Mol Biol Rev 67(1):16–37
Huvenne H, Smagghe G (2010) Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56(3):227–235
James C (2013) ISAAA Report on Global Status of Biotech/GM Crops, International Service for the Acquisition of Agri-Biotech Applications (ISAAA)
Mao Y-B, Cai W-J et al (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 25(11):1307–1313
Martin D, Maestro O et al (2006) RNAi studies reveal a conserved role for RXR in molting in the cockroach Blattella germanica. J Insect Physiol 52(4):410–416
Miller SC, Brown SJ et al (2008) Larval RNAi in Drosophila? Dev Genes Evol 218(9):505–510
Miska EA (2005) How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev 15(5):563–568
Napoli C, Lemieux C et al (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2(4):279–289
Niu Q-W, Lin S-S et al (2006) Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat Biotechnol 24(11):1420–1428
Ohnishi A, Hull JJ et al (2006) Targeted disruption of genes in the Bombyx mori sex pheromone biosynthetic pathway. Proc Natl Acad Sci USA 103(12):4398–4403
Ojha A, Sree KS et al (2014) Analysis of resistance to Cry1Ac in field-collected pink bollworm, Pectinophora gossypiella (Lepidoptera: Gelechiidae), populations. GM Crops Food 5(4):280–286
Ossowski S, Schwab R et al (2008) Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J 53(4):674–690
Pursel VG, Rexroad CE (1993) Status of research with transgenic farm animals. J Anim Sci 71(suppl 3):10–19
Quan GX, Kanda T et al (2002) Induction of the white egg 3 mutant phenotype by injection of the double-stranded RNA of the silkworm white gene. Insect Mol Biol 11(3):217–222
Rajagopal R, Arora N et al (2009) Resistance of Helicoverpa armigera to Cry1Ac toxin from Bacillus thuringiensis is due to improper processing of the protoxin. Biochem J 419:309–316
Roignant J-Y, Carre C et al (2003) Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA 9(3):299–308
Schneider CA, Rasband WS et al (2012) NIH Image to ImageJ: 25 years of image analysis.”. Nat Methods 9(7):671–675
Sharma A, Kumar S et al (2010) Bacillus thuringiensis protein Cry6B (BGSC ID 4D8) is toxic to larvae of Hypera postica. Curr Microbiol 62(2):597–605
Tabashnik BE, Van Rensburg JBJ et al (2009) Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol 102(6):2011–2025
Terenius O, Papanicolaou A et al (2011) RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol 57(2):231–245
Tomoyasu Y, Denell RE (2004) Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol 214(11):575–578
Turner CT, Davy MW et al (2006) RNA interference in the light brown apple moth, Epiphyas postvittana (Walker) induced by double stranded RNA feeding. Insect Mol Biol 15(3):383–391
Van der Krol AR, Mur LA et al (1990) Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 2(4):291–299
Vu TV, Roy Choudhury N et al (2012) Transgenic tomato plants expressing artificial microRNAs for silencing the pre-coat and coat proteins of a begomovirus, Tomato leaf curl New Delhi virus, show tolerance to virus infection. Virus Res 172(1):35–45
Wall RJ (1996) Transgenic livestock: progress and prospects for the future. Theriogenology 45(1):57–68
Zha W, Peng X et al (2011) Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens. PLoS ONE 6(5):e20504
Zhan X, Kawai S et al (1997) A new approach based on the leaf disc method for Agrobacterium mediated transformation and regeneration of aspen. Plant Sci 123(1):105–112
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31(13):3406–3415
Acknowledgments
We acknowledge the financial support from National Agriculture Innovation project (NAIP) of ICAR (Indian Council for Agricultural Research), India (Grant No. RNAi-2012). We acknowledge the assistance of Dr. Anil Sharma during the statistical analysis. We sincerely thank Tara Ram for technical assistance during rearing of H. armigera larvae.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Insect bioassays of transgenic tobacco plants with (a) wild type plant (b) amiR-24C construct (c) amiR-24 construct (TIFF 2377 kb)
Rights and permissions
About this article
Cite this article
Agrawal, A., Rajamani, V., Reddy, V.S. et al. Transgenic plants over-expressing insect-specific microRNA acquire insecticidal activity against Helicoverpa armigera: an alternative to Bt-toxin technology. Transgenic Res 24, 791–801 (2015). https://doi.org/10.1007/s11248-015-9880-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-015-9880-x