Abstract
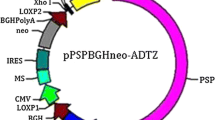
The feasibility of using the pig parotid secretory protein promoter to drive the β-glucanase transgene expression in mouse parotid glands was examined in this study. The parotid gland-specific vector expressing β-glucanase gene (GLU, from Paenibacillus polymyxa CP7) was constructed. Transgenic mice were produced by the pronuclear microinjection. Both PCR and Southern blot analysis showed that the mice carried the β-glucanase gene and the β-glucanase gene could be stably inherited. Furthermore, RT-PCR and northern blot analysis indicated that it was specifically expressed in the parotid. The β-glucanase activity in the saliva was found to be 0.18 U/mL. After feeding a diet containing 2 % β-glucan, the average daily gain of transgenic was significantly higher than non-transgenic mice. The crude protein and crude fat concentration in faeces of transgenic mice were significantly reduced compared with that of the non-transgenic mice. These results suggest that the successful expression of foreign β-glucanase in the animal parotid would offer a promising biological approach to reduce the anti-nutritional effect of β-glucans in feed.




Similar content being viewed by others
Abbreviations
- PSP:
-
Parotid secretory protein promoter
- GLU:
-
Glucanase
References
Ali S, Hall J, Hazlewood GP, Hirst BH, Gilbert HJ (1996) A protein targeting signal that functions in polarized epithelial cells in vivo. Biochem J 315(Pt 3):857–862
Ali S, Fontes CM, Hazlewood GP, Hirst BH, Clark AJ, Gilbert HJ, Hall J (1997) Co-integration and expression of bacterial and genomic transgenes in the pancreatic and intestinal tissues of transgenic mice. Gene 202:203–208
Allen ND, Cran DG, Barton SC, Hettle S, Reik W, Surani MA (1988) Transgenes as probes for active chromosomal domains in mouse development. Nature 333:852–855
al-Shawi R, Kinnaird J, Burke J, Bishop JO (1990) Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol 10:1192–1198
Bacic A, Stone BA (1981) Chemistry and organisation of aleurone cell wall components from wheat and barley. Aust J Plant Physiol 8:475–495
Bedford MR (1995) Mechanism of action and potential environmental benefits from the use of feed enzymes. Anim Feed Sci Technol 53:145–155
Bedford MR, Classen HL (1992) Reduction of intestinal viscosity through manipulation of dietary rye and pentosanase concentration is effected through changes in the carbohydrate composition of the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of broiler chicks. J Nutr 122:560–569
Bensadoun A, Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70:241–250
Brenes A, Smith M, Guenter W, Marquardt RR (1993) Effect of enzyme supplementation on the performance and digestive tract size of broiler chickens fed wheat- and barley-based diets. Poult Sci 72:1731–1739
Dygert S, Li LH, Florida D, Thoma JA (1965) Determination of reducing sugar with improved precision. Anal Biochem 13:367–374
Fan CL, Han XY, Xu ZR, Wang LJ, Shi LR (2009) Effects of beta-glucanase and xylanase supplementation on gastrointestinal digestive enzyme activities of weaned piglets fed a barley-based diet. J Anim Physiol Anim Nutr (Berl) 93:271–276
Fontes CM, Ali S, Gilbert HJ, Hazlewood GP, Hirst BH, Hall J (1999) Bacterial xylanase expression in mammalian cells and transgenic mice. J Biotechnol 72:95–101
Golovan SP, Hayes MA, Phillips JP, Forsberg CW (2001a) Transgenic mice expressing bacterial phytase as a model for phosphorus pollution control. Nat Biotechnol 19:429–433
Golovan SP, Meidinger RG, Ajakaiye A, Cottrill M, Wiederkehr MZ, Barney DJ, Plante C, Pollard JW, Fan MZ, Hayes MA, Laursen J, Hjorth JP, Hacker RR, Phillips JP, Forsberg CW (2001b) Pigs expressing salivary phytase produce low-phosphorus manure. Nat Biotechnol 19:741–745
Hall J, Hazlewood GP, Surani MA, Hirst BH, Gilbert HJ (1990) Eukaryotic and prokaryotic signal peptides direct secretion of a bacterial endoglucanase by mammalian cells. J Biol Chem 265:19996–19999
Hogan B, Constantini F, Lacy E (1986) Manipulating the mouse embryo. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Hogberg A, Lindberg JE, Leser T, Wallgren P (2004) Influence of cereal non-starch polysaccharides on ileo-caecal and rectal microbial populations in growing pigs. Acta Vet Scand 45:87–98
Jozefiak D, Rutkowski A, Jensen BB, Engberg RM (2006) The effect of beta-glucanase supplementation of barley- and oat-based diets on growth performance and fermentation in broiler chicken gastrointestinal tract. Br Poult Sci 47:57–64
Koller MM, Maeda N, Purushotham KR, Scarpace PJ, Humphreys-Beher MG (1992) A biochemical analysis of parotid and submandibular salivary gland function with age after simultaneous stimulation with pilocarpine and isoproterenol in female NIA Fischer 344 rats. Arch Oral Biol 37:219–230
Madsen HO, Hjorth JP (1985) Molecular cloning of mouse PSP mRNA. Nucleic Acids Res 13:1–13
McNab JM, Smithard RR (1992) Barley beta-glucan: an antinutritional factor in poultry feeding. Nutr Res Rev 5:45–60
Mikkelsen TR, Brandt J, Larsen HJ, Larsen BB, Poulsen K, Ingerslev J, Din N, Hjorth JP (1992) Tissue-specific expression in the salivary glands of transgenic mice. Nucleic Acids Res 20:2249–2255
Nahm KH, Carlson CW (1985) Effects of cellulase from Trichoderma viride on nutrient utilization by broilers. Poult Sci 64:1536–1540
Naruse K, Ishikawa H, Kawano HO, Ueda H, Kurome M, Miyazaki K, Endo M, Sawasaki T, Nagashima H, Makuuchi M (2005) Production of a transgenic pig expressing human albumin and enhanced green fluorescent protein. J Reprod Dev 51:539–546
Owerbach D, Hjorth JP (1980) Inheritance of a parotid secretory protein in mice and its use in determining salivary amylase quantitative variants. Genetics 95:129–141
Philip JS, Gilbert HJ, Smithard RR (1995) Growth, viscosity and beta-glucanase activity of intestinal fluid in broiler chickens fed on barley-based diets with or without exogenous beta-glucanase. Br Poult Sci 36:599–603
Rossiter P, Walsh G (2001) Stability of beta glucanase under conditions simulating the digestive tract. Biochem Soc Trans 29:A114
Samuelson LC (1996) Transgenic approaches to salivary gland research. Annu Rev Physiol 58:209–229
Shen MQ, Lu XX, Cui L, Xu JX (2003) Effects of different store condition on xylanase and β-glucanase activity. J Shanghai Jiaotong Univ Agric Sci 21:29–33
Sun J, Li W, Gu S (2002) Stability of beta glucanase under conditions simulating animal digestive tract in vitro. Chin J Anim Sci 38:18–19
Walsh GA, Power RF, Headon DR (1993) Enzymes in the animal-feed industry. Trends Biotechnol 11:424–430
White WB, Bird HR, Sunde ML, Prentice N, Burger WC, Marlett JA (1981) The viscosity interaction of barley beta-glucan with Trichoderma viride cellulase in the chick intestine. Poult Sci 60:1043–1048
Yin HF, Fan BL, Yang B, Liu YF, Luo J, Tian XH, Li N (2006) Cloning of pig parotid secretory protein gene upstream promoter and the establishment of a transgenic mouse model expressing bacterial phytase for agricultural phosphorus pollution control. J Anim Sci 84:513–519
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (973 Program, 2011CB944200), Chinese transgenic animal project (2011ZX08007-003 and 2011ZX08006-004). No additional external funding received for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Li-zheng Guan and Yu-ping Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guan, Lz., Sun, Yp., Xi, Qy. et al. β-Glucanase specific expression in the parotid gland of transgenic mice. Transgenic Res 22, 805–812 (2013). https://doi.org/10.1007/s11248-012-9682-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-012-9682-3