Abstract
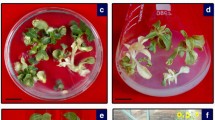
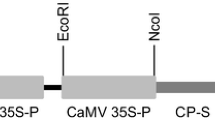
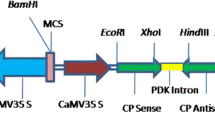
Sugarcane yellow leaf syndrome, characterized by a yellowing of the leaf midrib followed by leaf necrosis and growth suppression, is caused by sugarcane yellow leaf virus (SCYLV). We produced SCYLV-resistant transgenic sugarcane from a susceptible cultivar (H62-4671) and determined the amount of virus present following inoculation. The transgenic plants were produced through biolistic bombardment of cell cultures with an untranslatable coat protein gene. Presence of the transgene in regenerated plants was confirmed using PCR and Southern blot analysis. The transgenic lines were inoculated by viruliferous aphids and the level of SCYLV in the plants was determined. Six out of nine transgenic lines had at least 103-fold lower virus titer than the non-transformed, susceptible parent line. This resistance level, as measured by virus titer and symptom development, was similar to that of a resistant cultivar (H78-4153). The selected SCYLV-resistant transgenic sugarcane lines will be available for integration of the resistance gene into other commercial cultivars and for quantification of viral effects on yield.




Similar content being viewed by others
Abbreviations
- BA:
-
Benzyladenine
- IBA:
-
Indolebutyric acid
- MS:
-
Murashige and Skoog plant culture medium
- NAA:
-
Naphthaleneacetic acid, 2, 4-D: 2, 4-dichlorophenoxyacetic acid
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SCYLV:
-
Sugarcane yellow leaf virus
- YLS:
-
Yellow leaf syndrome
- Ubi:
-
Ubiquitin
- nptII:
-
Neomycin phosphotransferase
- SCYLVcp:
-
Sugarcane yellow leaf virus coat protein
- TPI:
-
Triosephosphate isomerase
References
Arencibia AD, Carmona ER, Tellez P, Chan M-T, Yu S-M, Trujillo LE, Oramas P (1998) An efficient protocol for sugarcane (Saccharum spp. L.) transformation mediated by Agrobacterium tumefaciens. Trans Res 7:213–222
Baulcome DC (1996) Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell 8:1833–1844
Borth W, Hu JS, Schenck S (1994) Double-stranded RNA associated with sugarcane yellow leaf syndrome. Sugar Cane 3:5–8
Bower R, Elliott AR, Potier BAM, Birch RG (1996) High-efficiency, microprojectile-mediated cotransformation of sugarcane, using visible or selectable markers. Mol Breed 2:239–249
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Comstock JC, Irvine JE, Miller FD (1994) Yellow leaf syndrome appears on the United States mainland. Sugar Cane 3:33–35
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: Version 2. Plant Mol Biol Report 1:19–22
Doyle J, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to a very high specific activity. Anal Biochem 132:6–13
Fitch M, Lehrer AT, Komor E, Moore PH (2001) Elimination of sugarcane yellow leaf virus from infected sugarcane plants by meristem tip culture visualized by tissue blot immunoassay. Plant Pathol 50:676–680
Gilbert RA, Glynn NC, Comstock JC, Davis MJ (2009) Agronomic performance and genetic characterization of sugarcane transformed for resistance to sugarcane yellow leaf virus. Field Crops Res 111:39–46
Ingelbrecht IL, Irvine JE, Mirkov TE (1999) Posttranscriptional gene silencing in transgenic sugarcane. Dissection of homology-dependent virus resistance in a monocot that has a complex polyploid genome. Plant Physiol 119:1187–1198
Lehrer AT, Komor E (2008) Symptom expression of yellow leaf disease in sugarcane cultivars with different degrees of infection by sugarcane yellow leaf virus. Plant Pathol 57:178–189
Lehrer AT, Schenck S, Yan S-L, Komor E (2007) Movement of aphid-transmitted sugarcane yellow leaf virus (ScYLV) within and between sugarcane plants. Plant Pathol 56:711–717
Lehrer AT, Wu K-K, Komor E (2009) Impact of sugarcane yellow leaf virus (SCYLV) on growth and sugar yield of sugarcane. J Gen Plant Pathol 75:288–296
Ma HM, Albert HH, Paull R, Moore PH (2000) Metabolic engineering of invertase activities in different subcellular compartments affects sucrose accumulation in sugarcane cells. Aust J Plant Physiol 27:1021–1030
Moonan F, Molina J, Mirkov TE (2000) Sugarcane yellow leaf virus: an emerging virus that has evolved by recombination between luteoviral and poleroviral ancestors. Virology 269:156–171
Moutia JFY, Saumtally S (1999) Symptomology of yellow leaf syndrome and detection and distribution of sugarcane yellow leaf virus in Mauritius. In: Singh V, Kumar V (eds) Proceedings of XXIII ISSCT congress, New Delhi 1999. STAI New Delhi, vol 2, pp 355–364
Murashige T, Skoog K (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Rassaby L, Girard JC, Letourmy P, Chaume J, Irey MS, Lockhart BE, Kodja H, Rott P (2003) Impact of sugarcane yellow leaf virus on sugarcane yield and juice quality in Reunion island. Eur J Plant Path 109:459–466
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Scagliusi SM, Lockhart BE (2000) Transmission, characterization and serology of a luteovirus associated with yellow leaf syndrome of sugarcane. Phytopathology 90:120–124
Schenck S, Lehrer AT (2000) Factors affecting the transmission and spread of sugarcane yellow leaf virus. Plant Dis 84:1085–1088
Schenck S, Hu JS, Lockhart BE (1997) Use of a tissue blot immunoassay to determine the distribution of sugarcane yellow leaf virus in Hawaii. Sugar Cane 4:5–8
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Vega J, Scagliusi SM, Ulian EC (1997) Sugarcane yellow leaf disease in Brazil: evidence for association with a luteovirus. Plant Dis 81:21–26
Wang MB, Abbott DC, Waterhouse PM (2000) A single copy of a virus-derived transgene encoding hairpin RNA gives immunity to barley yellow dwarf virus. Mol Plant Pathol 1:347–356
Xu J, Schubert J, Altpeter F (2001) Dissection of RNA-mediated ryegrass mosaic virus resistance in fertile transgenic perennial ryegrass (Lolium perenne L.). Plant J 26:265–274
Zhu YJ, Lim STS, Schenck S, Arcinas A, Komor E (2010) RT-PCR and quantitative real-time RT-PCR detection of sugarcane yellow leaf virus (SCYLV) in symptomatic and asymptomatic plants of Hawaiian sugarcane cultivars and the relation of SCYLV to yield. Eur J Plant Pathol 127:263–273
Acknowledgments
We thank Dr. E. Mirkov, Texas A&M Agriculture Experiment Station, Weslaco, TX for providing pFM395 construct; Dr. Henrik Albert at USDA, ARS for providing the pHA9 construct containing selection marker; Dr. M. Fitch, USDA, ARS, for advice on sugarcane transformation and clonal propagation and Dr. M. Irey, US Sugar, for providing primer sequences for SCYLV. This work was partially supported by a cooperative agreement (No. CA 58-5320-3-460) between the U.S. Department of Agriculture, Agricultural Research Service, and the Hawaii Agriculture Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y.J., McCafferty, H., Osterman, G. et al. Genetic transformation with untranslatable coat protein gene of sugarcane yellow leaf virus reduces virus titers in sugarcane. Transgenic Res 20, 503–512 (2011). https://doi.org/10.1007/s11248-010-9432-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9432-3