Abstract
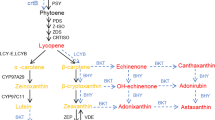
Plant chloroplasts are enriched in xanthophylls which participate in photosynthesis as light-absorbing pigments and as dissipaters of excess light. In comparison, chromoplasts have evolved the capacity to synthesize and store brightly coloured carotenoid pigments to give flowers and fruits the power to attract pollinators and fruit dispersers. The best performing accumulator of xanthophylls in tomato is the petal chromoplast in contrast to the fruit chromoplast which only seems able to store carotenes. We have generated genetically engineered tomato lines carrying the tomato CrtR-b2 transgene with the aim of forcing the fruit to accumulate beta-xanthophylls. Both chloroplast- and chromoplast-containing tissues of hemizygous transgenic plants were found to contain elevated xanthophyll contents as a direct consequence of the increased number of CrtR-b2 transcripts. Hemizygous transgenic leaves contained fourfold more violaxanthin than control leaves. Developing fruits were yellow instead of green since they lacked chlorophyll a, and their violaxanthin and neoxanthin contents were seven- and threefold higher, respectively, than those of the control. Ripe fruits of hemizygous transgenic plants contained free violaxanthin and significant amounts of esterified xanthophylls. Esterified xanthophylls were present also in ripe fruits of control and homozygous plants. However, in transgenic homozygous plants, we observed a reduction in transcript content in most tissues, particularly in petals, due to a post-transcriptional gene silencing process. These findings demonstrate that tomato fruit chromoplasts can accumulate xanthophylls with the same sequestration mechanism (esterification) as that exploited by chromoplasts of the tomato petal and pepper fruit. This study on transgenic plants overexpressing an important carotenoid gene (CrtR-b2) provides an interesting model for future investigations on perturbations in beta-carotene-derived xanthophyll synthesis which in turn may provide insights into the molecular mechanisms controlling carotenoid metabolism in tomato.


Similar content being viewed by others
References
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12:8711–8721
Bréhélin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12:260–266
Breithaupt DE, Bamedi A (2001) Carotenoid esters in vegetables and fruits: a screening with emphasis on β-cryptoxanthin esters. J Agric Food Chem 49:2064–2070
Britton G (1995) Structure and properties of carotenoids in relation to function. FASEB J 9:1551–1558
Britton G, Liaaen-Jensen S, Pfander H (eds) (2004) Carotenoids. Handbook. Compiled by Mercadante AZ, Egeland. ES Birkauser Verlag. Basel
D’Ambrosio C, Giorio G, Marino I, Merendino A, Petrozza A, Salfi L, Stigliani AL, Cellini F (2004) Virtually complete conversion of lycopene into β-carotene in fruits of tomato plants transformed with the tomato lycopene β-cyclase (tlcy-b) cDNA. Plant Sci 166:207–214
Davison PA, Hunter CN, Horton P (2002) Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418:203–206
DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57:711–738
Deruère J, Romer S, d’Harlingue A, Backhaus RA, Kuntz M, Camara B (1994) Fibril assembly and carotenoid overaccumulation in chromoplasts: a model for supramolecular lipoprotein structures. Plant Cell 6:119–133
Elmayan T, Vaucheret H (1996) Expression of single copies of a strongly expressed 35S transgene can be silenced post-transcriptionally. Plant J 9:787–797
Fraser PD, Pinto ME, Holloway DE, Bramley PM (2000) Technical advance: application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J 24:551–558
Fraser PD, Enfissi EMA, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM (2007) Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 19:3194–3211
Fraser PD, Enfissi EM, Bramley PM (2009) Genetic engineering of carotenoid formation in tomato fruit and the potential application of systems and synthetic biology approaches. Arch Biochem Biophys 483:196–204
Galpaz N, Ronen G, Khalfa Z, Zamir D, Hirschberg J (2006) A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. Plant Cell 18:1947–1960
Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J (2008) Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J 53:717–730
Giorio G, Stigliani AL, D’Ambrosio C (2007) Agronomic performance and transcriptional analysis of carotenoid biosynthesis in fruits of transgenic HighCaro and control tomato lines under field conditions. Transgenic Res 16:15–28
Giorio G, Stigliani AL, D’Ambrosio C (2008) Over-expression of carotene β-hydroxylase 1 (CrtR-b1) and lycopene β-cyclase (Lcy-b) in transgenic tomato fruits. Acta Hort 789:277–284
Hart JD, Scott KJ (1995) Development and evaluation of an HPLC method for the analysis of carotenoids in foods, and the measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chem 54:101–111
Howitt CA, Pogson BJ (2006) Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ 29:435–445
Jackson H, Braun CL, Ernst H (2008) The chemistry of novel xanthophyll carotenoids. Am J Cardiol 101:50D–57D
Jahns P, Latowski D, Strzalka K (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 1787:3–14
Kim J, DellaPenna D (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proc Natl Acad Sci USA 103:3474–3479
Kim J, Smith JJ, Tian L, DellaPenna D (2009) The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol 50:463–479
Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23:171–201
Lichtenthaler HK (2007) Biosynthesis, accumulation and emission of carotenoids, alpha-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynth Res 92:163–179
Lopez-Gomez R, Gomez-Lim MA (1992) A method for extracting intact RNA from fruits rich in polysaccharides using ripe mango mesocarp. Hortscience 27:440–442
North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50:810–824
Peterson DG, Boehm KS, Stack SM (1997) Isolation of milligram quantities of DNA from tomato (Lycopersicon esculentum) plant containing high levels of polyphenolic compounds. Plant Mol Biol Rep 15:148–153
Ralley L, Enfissi EM, Misawa N, Schuch W, Bramley PM, Fraser PD (2004) Metabolic engineering of ketocarotenoid formation in higher plants. Plant J 39:477–486
Rodriguez-Amaya BD (2001) A guide to carotenoid analysis in foods. ILSI Press, Washington D.C
Sandmann G, Romer S, Fraser PD (2006) Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metab Eng 8:291–302
Schubert D, Lechtenberg B, Forsbach A, Gils M, Bahadur S, Schmidt R (2004) Silencing in Arabidopsis T-DNA transformants: the predominant role of a gene-specific RNA sensing mechanism versus position effects. Plant Cell 16:2561–2572
Shewmaker C, Sheehy JA, Daley M, Colburn S, Ke DY (1999) Seed-specific overexpression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant J 20:401–412
Taylor KL, Brackenridge AE, Vivier MA, Oberholster A (2006) High-performance liquid chromatography profiling of the major carotenoids in Arabidopsis thaliana leaf tissue. J Chromatogr 1121:83–91
Tian L, DellaPenna D (2004) Progress in understanding the origin and functions of carotenoid hydroxylases in plants. Arch Biochem Biophys 430:22–29
van Roekel JSC, Damm B, Melchers LS, Hoekema A (1993) Factors influencing transformation frequency of tomato (Lycopersicon esculentum). Plant Cell Rep 12:644–647
Vaucheret H, Béclin C, Elmayan T, Feuerbach F, Godon C, Morel JB, Mourrain P, Palauqui JC, Vernhettes S (1998) Transgene-induced gene silencing in plants. Plant J 16:651–659
Vishnevetsky M, Ovadis M, Vainstein A (1999) Carotenoid sequestration in plants: the role of carotenoid-associated proteins. Trends Plant Sci 4:232–235
Acknowledgements
We gratefully acknowledge many colleagues of Metapontum Agrobios and Luciana Pace for their help. This project was conducted within the ITA.LYCO programme financed by the “Ministero dell’Istruzione, dell’Univerità e della Ricerca” of Italy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11248_2010_9387_MOESM1_ESM.doc
Fig. 1S Relative transcript content of psy1, psy2, CrtR-b1 and CrtR-b2 genes in various tissues of the three genotypes. The RT-qPCR analyses were performed with gene-specific TaqMan probes. Relative quantification of target gene transcripts was performed using the Standard curve method. Standard curves were prepared for both the target genes and the endogenous reference 18S rRNA gene Three replicated reactions were performed for each sample, both in the construction of the standard curve and in the quantification of samples. Relative starting quantities (RSQs) of transcripts were then divided by the corresponding 18S rRNA starting quantities to normalise for the amount of cDNA used for the PCR reactions. The derived Normalized Relative Quantities (NRQ) of transgenic UO and UU genotype samples were compared to that of the control OO sample (calibrator) across all gene-by-tissue combinations. The only exception was the leaf CrtR-b2 since this transcript is not detectable in the control OO sample. In this case, the UO sample was chosen as the calibrator. The estimates are expressed as the mean ± standard deviation. Fruits are at the Immature Green (IG) stage of control cv. Red Setter and CrtR-b2 transgenic plants (DOC 40 kb)
11248_2010_9387_MOESM2_ESM.doc
Fig. 2S HPLC profiles of carotenoids in ripe fruits of transgenic hemizygous genotype. (a) Unsaponified extract. (b) Saponified extract. Chromatograms were extracted at 450 nm. Peak identification: 1, cis-violaxanthin; 2, all-trans-violaxanthin; 3, neoxanthin; 4, luteoxanthin; 5, anteraxanthin; 6, lutein; 7, zeaxanthin; I.S., Internal Standard (trans-β-apo-8′-carotenal); 8, phytofluene; 9-10, neoxanthin ester; 11, all-trans- β-carotene; 12, δ-carotene derived; 13-14-15, lycopene isomers; 16, all-trans-lycopene. 17: 15-cis-lycopene (DOC 1678 kb)
Rights and permissions
About this article
Cite this article
D’Ambrosio, C., Stigliani, A.L. & Giorio, G. Overexpression of CrtR-b2 (carotene beta hydroxylase 2) from S. lycopersicum L. differentially affects xanthophyll synthesis and accumulation in transgenic tomato plants. Transgenic Res 20, 47–60 (2011). https://doi.org/10.1007/s11248-010-9387-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9387-4