Abstract
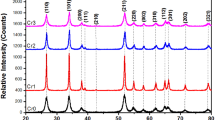
Ni(OH)2 doped with cerium cations was synthesized by a hydrothermal method and its electrochemical, photoelectrochemical and photocatalytic behavior was determined by the corresponding suitable techniques. Ni(OH)2 films doped with different Ce proportions were evaluated in a KOH support electrolyte solution by cyclic voltammetry. Scan-rate dependent voltammograms displayed pseudo-capacitive behavior while the charge transfer resistance of Ce-doped samples was determined through electrochemical impedance spectroscopy. The calculated charge transfer resistance value was 132 Ω for Ni(OH)2 containing 350 μmoles of Ce. Chronoamperometry under intermittent UV light was employed to measure the photo-response of nanomaterials. The experimental results indicated that the photocurrent of Ni(OH)2 containing 350 µmoles of Ce was ten times greater than that of pure Ni(OH)2. Photocatalytic activity of the powders was demonstrated under UV light irradiation accomplishing 83% of methyl orange degradation after 140 min of reaction with pseudo-first-order kinetics and the calculated degradation rate constant was 0.0125 min− 1. The results evidenced the electrochemical, photoelectrochemical and photocatalytic activity of the synthesized materials, which sets them as suitable materials for a wide range of promising photo-based applications.










Similar content being viewed by others
References
Chou S, Wang J, Chew SY, Liu HK, Dou SX (2008) Electrodeposition of MnO2 nanowires on carbon nanotube paper as free-standing, flexible electrode for supercapacitors. Electrochem Commun 10:1724–1727
Wu ZS, Ren W, Wang DW, Li F, Liu B, Cheng HM (2010) High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano 4:5835–5842. https://doi.org/10.1021/nn101754k
Hu CC, Chang KH, Lin MC, Wu YT (2006) Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett 6:2690–2695. https://doi.org/10.1021/nl061576a
Echresh A, Chey CO, Zargar Shoushtari M, Khranovskyy V, Nur O, Willander M (2015) UV photo-detector based on p-NiO thin film/n-ZnO nanorods heterojunction prepared by a simple process. J Alloys Compd 632:165–171. https://doi.org/10.1016/j.jallcom.2015.01.155
Sun W, Xiao L, Wu X (2019) Facile synthesis of NiO nanocubes for photocatalysts and supercapacitor electrodes. J Alloys Compd 772:465–471. https://doi.org/10.1016/j.jallcom.2018.09.185
Chou S, Wang J, Liu HK, Dou SX (2008) Electrochemical deposition of porous Co(OH)2 nanoflake films on stainless steel mesh for flexible supercapacitors. J Electrochem Soc 155:A926–A929
Motahari F, Mozdianfard MR, Soofivand F, Salavati-Niasari M (2014) NiO nanostructures: synthesis, characterization and photocatalyst application in dye wastewater treatment. RSC Adv 4:27654–27660. https://doi.org/10.1039/c4ra02697g
Zhang X, Gu A, Wang G, Fang B, Yan Q, Zhu J, Sun T, Ma J, Hoon Hng H (2011) Enhanced electrochemical catalytic activity of new nickel hydroxide nanostructures with (100) facet. CrystEngComm 13:188–192. https://doi.org/10.1039/c003791p
Wang X, Luo H, Parkhutik PV, Millan AC, Matveeva E (2003) Studies of the performance of nanostructural multiphase nickel hydroxide. J Power Sources 115:153–160
Akinc M, Jongen N, Lemaitre J, Hofmann H (1998) Synthesis of nickel hydroxide powders by urea decomposition. J Eur Ceram Soc 18:1559–1564
Jayalakshmi M, Radhika P, Phani Raja K, Mohan Rao M (2007) Solvent and thiourea adsorption/intercalation effects on the solid-state electrochemistry of a-phase nickel hydroxide nanoparticles. J Solid State Electrochem 11:165–172. https://doi.org/10.1007/s10008-005-0081-z
Srinivasan V, Weidner JW (1997) An electrochemical route for making porous nickel oxide electrochemical capacitors. J Electrochem Soc 144:L210–L213
Li M, Xu S, Zhu Y, Yang P, Wang L, Chu PK (2014) Heterostructured Ni(OH)2-Co(OH)2 composites on 3D ordered Ni-Co nanoparticles fabricated on microchannel plates for advanced miniature supercapacitor. J Alloys Compd 589:364–371. https://doi.org/10.1016/j.jallcom.2013.11.230
Zhang Y, Zhao Y, An W, Xing L, Gao Y, Liu J (2017) Heteroelement Y-doped α-Ni(OH)2 nanosheets with excellent pseudocapacitive performance. J Mater Chem A 5:10039–10047. https://doi.org/10.1039/c7ta00963a
Adekunle AS, Oyekunle JAO, Oluwafemi OS, Joshua AO, Makinde WO, Ogunfowokan AO, Eleruja MA, Ebenso EE (2014) Comparative catalytic properties of Ni(OH)2 and NiO nanoparticles towards the degradation of nitrite (NO2–) and nitric oxide (NO). Int J Electrochem Sci 9:3008–3021
Cai X, Cai Y, Liu Y, Deng S, Wang Y, Wang Y, Djerdj I (2014) Photocatalytic degradation properties of Ni(OH)2 nanosheets/ZnO nanorods composites for azo dyes under visible-light irradiation. Ceram Int 40:57–65. https://doi.org/10.1016/j.ceramint.2013.05.103
Wang YM, Zhao DD, Zhao YQ, Xu CL, Li HL (2012) Effect of electrodeposition temperature on the electrochemical performance of a Ni(OH)2 electrode. RSC Adv 2:1074–1082. https://doi.org/10.1039/c1ra00613d
Mao Y, Yang H, Chen J, Chen J, Tong Y, Wang X (2014) Significant performance enhancement of ZnO photoanodes from Ni(OH)2 electrocatalyst nanosheets overcoating. Nano Energy 6:10–18. https://doi.org/10.1016/j.nanoen.2014.02.008
Ranjit KT, Willner I, Bossmann SH, Braun AM (2001) Lanthanide oxide doped titanium dioxide photocatalysts: effective photocatalysts for the enhanced degradation of salicylic acid and t-cinnamic acid. J Catal 204:305–313
Fu C, Li T, Qi J, Pan J, Chen S, Cheng C (2010) Theoretical study on the electronic and optical properties of Ce3+-doped TiO2 photocatalysts. Chem Phys Lett 494:117–122. https://doi.org/10.1016/j.cplett.2010.05.085
Muthukumaran P, Chikkili VR, Sumathi C, Slairaj D, Rameshthangam P, Wilson J, Sathish R, Subbiah A (2016) Cerium doped nickel-oxide nanostructures for riboflavin biosensing and antibacterial applications. New J Chem 40:2741–2748. https://doi.org/10.1039/c5nj03539b
Liu C, Tang X, Mo C, Qiang Z (2008) Characterization and activity of visible-light-driven TiO2 photocatalyst codoped with nitrogen and cerium. J Solid State Chem 181:913–919. https://doi.org/10.1016/j.jssc.2008.01.031
Regmi C, Maya-Flores E, Lee SW, Rodríguez-González V (2018) Cerium-doped β-Ni(OH)2 hexagon nanosheets: an effective photocatalyst for the degradation of the emerging water pollutant, naproxen. Nanotechnology 29:375603. https://doi.org/10.1088/1361-6528/aace14
Iyyappa Rajan P, Judith Vijaya J, Jesudoss SK, Kaviyarasu K, John Kennedy L, Jothiramalingam R, Al-Lohedan HA, Vaali-Mohammed MA (2017) Green-fuel-mediated synthesis of self-assembled NiO nano-sticks for dual applications—photocatalytic activity on Rose Bengal dye and antimicrobial action on bacterial strains. Mater Res Express 4:08503
Sonavane AC, Inamdar AI, Shinde PS, Deshmukh HP, Patil RS, Patil PS (2010) Efficient electrochromic nickel oxide thin films by electrodeposition. J Alloys Compd 489:667–673. https://doi.org/10.1016/j.jallcom.2009.09.146
Abd AN, Ali RS, Hussein AA (2016) Fabrication and characterization of nickel heterojunction oxide nanoparticles/silicon. J Multidiscip Eng Sci Stud 2:434–440
Mohammad Shiria H, Aghazadeh M (2012) Synthesis, characterization and electrochemical properties of capsule-like NiO nanoparticles. J Electrochem Soc 159:E132–E138
Sabouri Z, Akbari A, Hosseini HA, Darroudi M (2018) Facile green synthesis of NiO nanoparticles and investigation of dye degradation and cytotoxicity effects. J Mol Struct 1173:931–936
Rodríguez-González V, Marceau E, Che M, Pepe C (2007) Influence of the morphology and impurities of Ni(OH)2 on the synthesis of neutral Ni(II)–amino acid complexes. J Solid State Chem 180:3469–3478
Oliver-Tolentino MA, Vázquez-Samperio J, Manzo-Robledo A, González-Huerta RG, Flores-Moreno JL, Ramírez-Rosales D, Guzmán-Vargas A (2014) An approach to understanding the electrocatalytic activity enhancement by superexchange interaction toward OER in alkaline media of Ni-Fe LDH. J Phys Chem C 118:22432–22438. https://doi.org/10.1021/jp506946b
Choudhury B, Borah B, Choudhury A (2012) Extending photocatalytic activity of TiO2 nanoparticles to visible region of illumination by doping of cerium. Photochem Photobiol 88:257–264. https://doi.org/10.1111/j.1751-1097.2011.01064.x
Jiang Y, Chen D, Song J, Jiao Z, Ma Q, Zhang H, Cheng L, Zhao B, Chu Y (2013) A facile hydrothermal synthesis of graphene porous NiO nanocomposite and its application in electrochemical capacitors. Electrochim Acta 91:173–178
Zhu Z, Ping J, Huang X, Hu J, Chen Q, Ji X, Banks CE (2012) Hexagonal nickel oxide nanoplate-based electrochemical supercapacitor. J Mater Sci 47:503–507. https://doi.org/10.1007/s10853-011-5826-8
Wang J (2006) Analytical electrochemisitry: controlled-potential techniques, 3rd edn. Wiley, Hoboken
Liu C, Yu Z, Neff D, Zhamu A, Jang BZ (2010) Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett 10:4863–4868. https://doi.org/10.1021/nl102661q
Zheng X, Duan S, Liu S, Wei M, Xia F, Tian D, Zhou C (2015) Sensitive and simultaneous method for the determination of naphthol isomers by an amino-functionalized, SBA-15-modified carbon paste electrode. Anal Methods 7:3063–3071. https://doi.org/10.1039/c5ay00027k
Tian H, Wan C, Zheng W, Hu X, Qiao L, Wang X (2016) Construction of a ternary hybrid of CdS nanoparticles loaded on mesoporous-TiO2/RGO for the enhancement of photocatalytic activity. RSC Adv 6:84722–84729. https://doi.org/10.1039/c6ra16094h
Liu S, Xu YJ (2016) Photo-induced transformation process at gold clusters-semiconductor interface: implications for the complexity of gold clusters-based photocatalysis. Sci Rep 6:1–13. https://doi.org/10.1038/srep22742
Long M, Jiang J, Li Y, Cao R, Zhang L, Cai W (2011) Effect of gold nanoparticles on the photocatalytic and photoelectrochemical performance of au modified BiVO4. Nano-Micro Lett 3:171–177. https://doi.org/10.3786/nml.v3i3.p171-177
Rebello A, Adeyeye AO (2016) Robust electric-field tunable opto-electrical behavior in Pt-NiO-Pt planar structures. Sci Rep 6:1–8. https://doi.org/10.1038/srep28007
Luo Y, Qiao L, Zhang Q, Xu H, Shen Y, Lin Y, Nan C (2018) Tunable photoelectric response in NiO-based heterostructures by various orientations. Appl Phys Lett 112:093301
Lv H, Wang C, Li G, Burke R, Krauss TD, Gao Y, Eisenberg R (2017) Semiconductor quantum dot-sensitized rainbow photocathode for effective photoelectrochemical hydrogen generation. PNAS 114:11297–11302
Ramasamy P, Kwak D, Lim DH, Ra HS, Lee JS (2016) Solution synthesis of GeS and GeSe nanosheets for high-sensitivity photodetectors. J Mater Chem C 4:479–485. https://doi.org/10.1039/c5tc03667d
Yan J, Gu J, Wang X, Fan Y, Zhao Y, Lian J, Xu Y, Song Y, Xu H, Li H (2017) Design of 3D WO3/h-BN nanocomposites for efficient visible-light-driven photocatalysis. RSC Adv 7:25160–25170. https://doi.org/10.1039/c7ra02929b
Fang W, Zhou Y, Dong C, Xiang M, Zhang J (2016) Enhanced photocatalytic activities of vacuum activated TiO2 catalysts with Ti3+ and N co-doped. Catal Today 266:188–196
Jiang Z, Wan W, Wei W, Chen K, Li H, Wong PK, Xie J (2017) Gentle way to build reduced titanium dioxide nanodots integrated with graphite-like carbon spheres: From DFT calculation to experimental measurement. Appl Catal B Environ 204:283–295
Tian H, Teng F, Xu J, Lou S, Li N, Zhao Y, Chen M (2015) An innovative anion regulation strategy for energy bands of semiconductors: a case from bi 2 o 3 to bi 2 O(OH) 2 so 4. Sci Rep 5:1–9. https://doi.org/10.1038/srep07770
Kadam LD, Patil PS (2001) Studies on electrochromic properties of nickel oxide thin films prepared by spray pyrolysis technique. Sol Energy Mater Sol Cells 69:361–369
Maciak E, Opilski Z (2007) Transition metal oxides covered Pd film for optical H2 gas detection. Thin Solid Films 515:8351–8355. https://doi.org/10.1016/j.tsf.2007.03.022
He J, Lindström H, Hagfeldt A, Lindquist SE (1999) Dye-sensitized nanostructured p-type nickel oxide film as a photocathode for a solar cell. J Phys Chem B 103:8940–8943. https://doi.org/10.1021/jp991681r
Fu X, Huang D, Qin Y, Li L, Jiang X, Chen S (2014) Effects of preparation method on the microstructure and photocatalytic performance of ZnSn(OH)6. Appl Catal B Environ 148–149:532–542
Wang J, Li H, Meng S, Zhang L, Fu X, Chen S (2017) One-pot hydrothermal synthesis of highly efficient SnOx/Zn2SnO4 composite photocatalyst for the degradation of methyl orange and gaseous benzene. Appl Catal B Environ 200:19–30
Znad H, Abbas K, Hena S, Awual MR (2018) Synthesis a novel multilamellar mesoporous TiO2/ZSM-5 for photo-catalytic degradation of methyl orange dye in aqueous media. J Environ Chem Eng 6:218–227. https://doi.org/10.1016/j.jece.2017.11.077
Nguyen CH, Fu CC, Juang RS (2018) Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: efficiency and degradation pathways. J Clean Prod 202:413–427. https://doi.org/10.1016/j.jclepro.2018.08.110
Saien J, Mesgari Z (2017) Photocatalytic degradation of methyl orange using hematoporphyrin/N-doped TiO2 nanohybrids under visible light: Kinetics and energy consumption. Appl Organomet Chem 31:1–11. https://doi.org/10.1002/aoc.3755
Lv T, Pan L, Liu X, Sun Z (2012) Visible-light photocatalytic degradation of methyl orange by CdS-TiO2-Au composites synthesized via microwave-assisted reaction. Electrochim Acta 83:216–220. https://doi.org/10.1016/j.electacta.2012.08.018
Gupta VK, Saravanan R, Agarwal S, Gracia F, Khan MM, Qin J, Mangalaraja RV (2017) Degradation of azo dyes under different wavelengths of UV light with chitosan-SnO2 nanocomposites. J Mol Liq 232:423–430. https://doi.org/10.1016/j.molliq.2017.02.095
Ökte AN, Yilmaz Ö (2008) Photodecolorization of methyl orange by yttrium incorporated TiO2 supported ZSM-5. Appl Catal B Environ 85:92–102
Arabi M, Baizaee SM, Bahador A, Otaqsara SMT (2018) Rapid, controllable, one-pot and room-temperature aqueous synthesis of ZnO:Cu nanoparticles by pulsed UV laser and its application for photocatalytic degradation of methyl orange. Luminescence 33:475–485. https://doi.org/10.1002/bio.3436
Acknowledgements
C. Martínez-Sánchez appreciates the Postdoctoral scholarship granted by CONACYT. We gratefully acknowledge G. Labrada-Delgado and H. Silva-Pereyra from LINAN-IPICYT for the FESEM and TEM characterizations. V. Rodríguez-González is grateful to KOFTS for the Brain Pool Program No. 152S-2-31424. This research was partially supported by the National Research of Korea (NRF) funded by the Ministry of Education, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martínez-Sánchez, C., Regmi, C., Lee, S.W. et al. Effects of Ce Doping on the Photocatalytic and Electrochemical Performance of Nickel Hydroxide Nanostructures. Top Catal 64, 73–83 (2021). https://doi.org/10.1007/s11244-020-01295-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01295-y