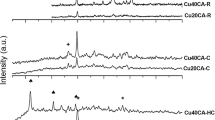
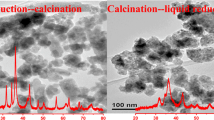
Abstract
Cu–Co-based catalysts derived from hydrotalcite (HT)-type precursors were applied in higher alcohol synthesis (HAS) at 280 °C, 60 bar and a H2/CO ratio of 1/1. Catalysts with higher Cu/Co ratios were found to provide the best trade-off between selective alcohol formation and moderate Fischer–Tropsch synthesis (FTS) activity. Within the alcohols and hydrocarbons formed the productivities decreased exponentially with increasing chain length according to the ASF distribution indicating a chain growth mechanism. Thermal analysis revealed the presence of different bivalent cations in one single HT-type precursor phase. After calcination at lower temperatures (Tcalc < 600 °C) a carbonate-modified ZnAl2O4 matrix was obtained. Within this amorphous matrix Cu2+ and Co2+ were found to be partially embedded resulting in an impeded ion reduction. After HAS the presence of bulk Co2C was detected by XRD. Both close contact of Cu0 and Co0 as well as Co2C–Co0 interfaces are known to provide the mechanistic requirements for higher alcohol formation. For comparison HAS was performed over a physical mixture consisting of the Al-containing HTs of Cu, Co or Zn. For the simultaneously co-precipitated samples the major roles of Cu are to decrease the FTS activity of metallic Co and to lower the alcohol chain growth probability by intimate Cu0–Co0 interactions. With increasing Cu content the alcohol selectivities were found to increase at the expense of high conversion, with ethanol being the major oxygenate product for all HT-based catalysts.






Similar content being viewed by others
References
Tsai Y-T, Mo X, Campos A, Goodwin JG Jr, Spivey JJ (2011) Appl Catal A 396:91
Nunan JG, Herman RG, Klier K (1989) J Catal 116:222
Baker JE, Burch R, Golunski SE (1989) Appl Catal A 53:279
Courty P, Durand D, Freund E, Sugier A (1982) J Mol Catal 17:241
Marchi AJ, Di Cosimo JI, Apesteguía CR (1992) Catal Today 15:383
Behrens M, Kasatkin I, Kühl S, Weinberg G (2010) Chem Mater 22:386
Kühl S, Tarasov A, Zander S, Kasatkin I, Behrens M (2014) Chem Eur J 20:3782
Zhu K, Liu C, Ye X, Wu Y (1998) Appl Catal A 168:365
Turco M, Bagnasco G, Costantino U, Marmottini F, Montanari T, Ramis G, Busca G (2004) J Catal 228:43
Busca G, Costantino U, Marmottini F, Montanari T, Patrono P, Pinzari F, Ramis G (2006) Appl Catal A 310:70
Costantino U, Marmottini F, Sisani M, Montanari T, Ramis G, Busca G, Turco M, Bagnasco G (2005) Solid State Ionics 176:2917
Espinal R, Taboada E, Molins E, Chimentao RJ, Medina F, Llorca J (2012) RSC Adv 2:2946
Ginés MJL, Amadeo N, Laborde M, Apesteguía CR (1995) Appl Catal A 131:283
Nishida K, Atake I, Li D, Shishido T, Oumi Y, Sano T, Takehira K (2008) Appl Catal A 337:48
Cavani F, Trifirò F, Vaccari A (1991) Catal Today 11:173
Spivey JJ, Egbebi A (2007) Chem Soc Rev 36:1514
Subramani V, Gangwal SK (2008) Energy Fuels 22:814
Anton J, Ruland H, Kaluza S, Muhler M (2015) Catal Lett 145:1374
Rives V, Dubey A, Kannan S (2001) Phys Chem Chem Phys 3:4826
Alejandre A, Medina F, Rodriguez X, Salagre P, Sueiras JE (1999) J Catal 188:311
Kannan S, Bevy LP (eds) (2006) Trends in catalysis research: hydrotalcites as potential catalysts for hydroxylation of phenol. Nova Science Publishers, New York
Velu S, Suzuki K, Hashimoto S, Satoh N, Ohashi F, Tomura S (2001) J Mater Chem 11:2049
Kannan S, Swamy CS (1992) J Mater Sci Lett 11:1585–1587
Liu Q, Wang B, Wang C, Tian Z, Qu W, Ma H, Xu R (2014) Green Chem 16:2604
Montanari T, Sisani M, Nocchetti M, Vivani R, Delgado MCH, Ramis G, Busca G, Costantino U (2010) Catal Today 152:104
Alejandre A, Medina F, Salagre P, Correig X, Sueiras JE (1999) Chem Mater 11:939
Lwin Y, Mohamad A, Yaakob Z, Daud W (2000) React Kinet Catal L 70:303–310
Anton J, Nebel J, Song H, Froese C, Weide P, Ruland H, Muhler M, Kaluza S (2016) J Catal 335:175–186
Günter MM, Ressler T, Jentoft RE, Bems B (2001) J Catal 203:133
Naumann d’Alnoncourt R, Xia X, Strunk J, Löffler E, Hinrichsen O, Muhler M (2006) Phys Chem Chem Phys 8:1525
Kasatkin I, Kurr P, Kniep B, Trunschke A, Schlögl R (2007) Angew Chem Int Ed 119:7465
Behrens M, Furche A, Kasatkin I, Trunschke A, Busser W, Muhler M, Kniep B, Fischer R, Schlögl R (2010) Chem Cat Chem 2:816
Volkova G, Yurieva T, Plyasova L, Naumova M, Zaikovskii V (2000) J Mol Catal A 158:389
Pei Y-P, Liu J-X, Zhao Y-H, Ding Y-J, Liu T, Dong W-D, Zhu H-J, Su H-Y, Yan L, Li J-L, Li W-X (2015) ACS Catal 5:3620
Xiaoding X, Scholten JJF, Mausbeck D (1992) Appl Catal A 82:91
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anton, J., Nebel, J., Göbel, C. et al. CO Hydrogenation to Higher Alcohols over Cu–Co-Based Catalysts Derived from Hydrotalcite-Type Precursors. Top Catal 59, 1361–1370 (2016). https://doi.org/10.1007/s11244-016-0663-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-016-0663-2