Abstract
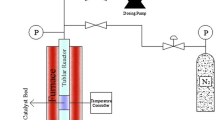
Arrays of nickel phosphide nanorods were successfully synthesized by nanocasting using mesostructured silica SBA-15 as a hard template (HT-Ni2P). After temperature-programmed reduction of the phosphate precursor infiltrated within the pore walls of SBA-15, the unsupported material was obtained by removing the silica matrix with diluted HF. The pore channel of the SBA-15 template stabilizes the Ni2P particles, preventing sintering after the high reduction temperature and shaping their elongated morphology. Moreover, HT-Ni2P catalyst shows an improvement in the textural properties with a significantly higher surface area than the reference sample synthesized in the absence of template. X-ray diffraction revealed that the only crystalline phase present in this material was Ni2P. On the other hand, transmission electron microscopy shows that the catalyst is mainly constituted by agglomerates of nanorods. Through EDX microanalysis the efficient removal of silicon was confirmed. Under hydrotreating conditions, nanorods of Ni2P show a fourfold enhancement in the conversion of methyl oleate with respect to conventional Ni2P synthesized in absence of hard template. Nevertheless, when these data are normalized to surface area, the specific activity of HT-Ni2P nanorods is significantly lower than that of the conventionally prepared sample. Differences in selectivity were also observed as Ni2P nanorods favored the decarboxylation reaction leading to a higher yield of n-heptadecane.






Similar content being viewed by others
References
Hicks JC (2011) J Phys Chem Lett 2:2280–2287
Furimsky E (2000) Appl Catal A 199(2):147–190
Vispute TP, Zhang H, Sanna A, Xiao R, Huber GW (2010) Science 330:1222
Knothe G (2010) Prog Energy Combust. 36:364–376
Kubickova I, Snare M, Eranen K, Maki-Arvela P, Murzin DY (2005) Catal Today 106:197
Boda L, Onyestya G, Solt H, Lonyi F, Valyon J, Thernesz A (2010) Appl Catal A 374:158
Kubicka D, Kaluz L (2010) Appl Catal A 372:199
Kubicka D, Horácek J (2011) Appl Catal A 394:9
Murata K, Liu Y, Inaba M, Takahara I (2010) Energy Fuels 24:2404
Zhao HY, Li D, Bui P, Oyama ST (2011) Appl Catal A 391:305
Whiffen VML, Smith KJ (2010) Energy Fuel 24:4728
Li K, Wang R, Chen J (2011) Energy Fuels 25:854–863
Yang YX, Ochoa-Hernández C, de la Peña O’Shea VA, Coronado JM, Serrano DP (2012) ACS Catal. 2(4):592–598
Stinner C, Prins R, Weber Th (2001) J Catal 202:187–194
Yang PF, Jiang ZX, Ying PL, Li C (2008) J Catal 253:66–73
Yang SF, Liang CH, Prins R (2006) J Catal 241:465–469
Wang R, Smith KJ (2010) Appl Catal A 380:149–164
Kim HY, Ma XL, Song CS (2005) Energy Fuel 19:353
Yang SF, Liang CH, Prins R (2006) J Catal 237:118–130
Gaudette AF, Burns AW, Hayes JR, Smith MC, Bowker RH, Seda T, Bussell ME (2010) J Catal 272:18
Oyama ST, Lee YK (2008) J Catal 258:393
Oyama ST, Gott T, Zhao H, Lee Y-K (2009) Catal Today 143:94
Zhao D, Yang P, Huo Q, Chmelka BF, Stucky GD (1998) Curr Opin Solid State Mater Sci 3:111–121
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Science 279:548–552
Li K, Wang R, Chen J (2011) Energy Fuels 25(3):854–863
Koranyi TI, Coumans AE, Hensen EJM, Ryoo R, Kim HS, Pfeifer E, Kasztovszky Z (2009) Appl Catal A 365:48–54
Acknowledgments
This study has received financial support of the RESTOENE and LIGCATUP program funded by, respectively, the Consejería de Educación of Comunidad de Madrid and the Spanish Ministry of Science and Competiveness. YXY and VPO thank the financial support of, respectively, AMAROUT (FP7-PEOPLE) and “Ramón y Cajal” (MICINN) programs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Ochoa-Hernández, C., Pizarro, P. et al. Synthesis of Nickel Phosphide Nanorods as Catalyst for the Hydrotreating of Methyl Oleate. Top Catal 55, 991–998 (2012). https://doi.org/10.1007/s11244-012-9886-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-012-9886-z