Abstract
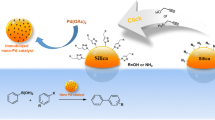
Cinchonidine, a naturally occurring cinchona alkaloid, was tethered to a high-surface-area silica substrate in order to create a new solid chiral catalyst. Two synthetic routes were explored for this grafting, relying on the use of an intermediate linker and so-called “click” chemistry. Both routes proved viable, but the procedure where cinchonidine is first derivatized with 3-isocyanatopropyltriethoxysilane (ICPTEOS) at the alcohol position and the resulting product then anchored to the silica surface was deemed the most efficient. Bonding to the surface occurs via the formation of Si–O–Si bonds, on average two out of the three possible per cinchonidine, and takes place preferentially at silica surface sites with two geminal hydroxyl groups on the same silicon atom. Approximately 10% of the available OH surface groups are derivatized in this procedure. The resulting catalyst was successfully tested for the addition of aromatic thiols to unsaturated ketones, a reaction promoted by amines (the tertiary quinuclidine nitrogen atom in the case of cinchonidine). The activity of the supported cinchonidine proved comparable to that of the free molecule, but tethering does lead to a significant loss in enantioselectivity.






Similar content being viewed by others
References
Kaufman TS, Rúveda EA (2005) Angew Chem Int Ed 44:854
Ashley E, McGready R, Proux S, Nosten F (2006) Travel Med Infect Dis 4:159
White NJ (2007) Lancet Infect Dis 7:549
Meyer CG, Marks F, Hay J (2004) Trop Med Int Health 9:1239
Lämmerhofer M, Lindner W (2008) Adv Chromatog 46:1
Wynberg H (1986) Top Stereochem 16:87
Kacprzak K Gawroński J (2001) Synthesis 961
Mallat T, Orglmeister E, Baiker A (2007) Chem Rev 107:4863
Ma Z, Zaera F (2009) Chiral modification of catalytic surfaces. In: Ozkan US (ed) Design of heterogeneous catalysis: new approaches based on synthesis, characterization, and modelling. Wiley-VCH, Weinheim, pp 113–140
Notestein JM, Katz A (2006) Chem Eur J 12:3954
Copéret C, Basset J-M (2007) Adv Synth Catal 349:78
Corma A, Garcia H (2006) Adv Synth Catal 348:1391
Margelefsky EL, Zeidan RK, Davis ME (2008) Chem Soc Rev 37:1118
Kolb HC, Finn MG, Sharpless KB (2001) Angew Chem Int Ed 40:2004
Kim G-J, Shin J-H (1999) Tetrahedron Lett 40:6827
Choong ES (2005) Annu Rep Prog Chem C 101:143
Hiemstra H, Wynberg H (1981) J Am Chem Soc 103:417
Tian S-K, Chen Y, Hang J, Tang L, McDaid P, Deng L (2004) Acc Chem Res 37:621
Hellriegel C, Skogsberg U, Albert K, Lämmerhofer M, Maier NM, Lindner W (2004) J Am Chem Soc 126:3809
Kacprzak KM, Maier NM, Lindner W (2006) Tetrahedron Lett 47:8721
Iglesias-Hernández M, Sánchez-Alonso F (2000). In: Corma A, Melo FV, Mendioroz S, José Luis GF (eds) Studies in surface science and catalysis, vol 130. Elsevier, Amsterdam, pp 3393–3398
Corma A, Iborra S, Rodríguez I, Iglesias M, Sánchez F (2002) Catal Lett 82:237
Tertykh VA, Yanishpolskii VV, Bereza LV, Pesek JJ, Matyska M (2000) J Therm Anal Calorim 62:539
Lindholm A, Maki-Arvela P, Toukoniitty E, Pakkanen TA, Hirvi JT, Salmi T, Murzin DY, Sjoholm R, Leino R (2002) J Chem Soc Perkin Trans 1:2605
Busygin I, Toukoniitty E, Sillanpää R Murzin Leino R (2005) Eur J Org Chem 2811
Reyes P, Campos C, Fierro JLG (2007) J Chil Chem Soc 52:1249
Prideaux EBR, Winfield FT (1930) Analyst 55:561
Socrates G (1994) Infrared characteristic group frequencies: tables and charts. Wiley, Chichester
Chu W, LeBlanc RJ, Williams CT, Kubota J, Zaera F (2003) J Phys Chem B 107:14365
Khalil AM (1981) Surf Technol 14:383
Moreland CG, Philip A, Carroll FI (1974) J Org Chem 39:2413
Caravajal GS, Leyden DE, Quinting GR, Maciel GE (1988) Anal Chem 60:1776
Krupczyńska K, Buszewski B, Jandera P (2004) Anal Chem 76(13):226A–234A
Lai J, Ma Z, Mink L, Mueller LJ, Zaera F (2009) J Phys Chem B 113:11696
Zaera F (2009) Acc Chem Res 42:1152
Zaera F (2008) J Phys Chem C 112:16196
Acknowledgements
Funds for this research were provided by a grant from the US Department of Energy. We also thank Dr. Dan Borchardt and the UCR Analytical Chemistry Instrumentation Facility (ACIF) for help with the acquisition of the solid-state NMR data. The NMR spectrometer used for those experiments was purchased with funds from grants from the US National Science Foundation (NSF CHE-0541848) and the US National Institute of Health (NIH S10RR023677).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, J., Lee, I. & Zaera, F. Cinchona Alkaloids Tethered on Porous Silica as Enantioselective Heterogeneous Catalysts. Top Catal 54, 1340–1347 (2011). https://doi.org/10.1007/s11244-011-9760-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-011-9760-4