Abstract
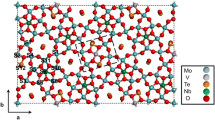
The M1 phase of the MoV(Nb,Ta)TeO system is one of the most effective catalysts for the ammoxidation and selective oxidation of propane to acrylonitrile (AN) and acrylic acid, respectively. The active centers of the M1 phase reside on the ab planes of this crystalline material (i.e., the (001) lattice face). Early on we proposed that the thus located active centers contain all key catalytic elements strategically placed for the conversion of propane to AN. These seven element comprising active centers contain: five metal oxide octahedra (2 V 5+0.32 /Mo 6+0.68 , 1 V 4+0.62 /Mo 6+0.38 , 2Mo 6+0.5 /Mo 5+0.5 ) and two Te4+—oxygen sites. In this contribution we analyze the various compositional probabilities of the seven element active centers and their additional eight element surroundings and conclude that there are 32 possible compositional arrangements of this 15 element assembly. From the diverse structural arrangements, diverse catalytic properties can be assigned to the individual sites, leading to diverse propane reaction pathways. We conclude that there are 22% AN forming, 22% propylene, 10% waste and 46% inert sites. After normalization these sites are deemed to lead to the following product yields: 41% AN, 41% propylene and 18% waste. The highest experimentally attained AN yield from propane is 42%, employing M1 phase only, which coincides with the predicted value of a concerted mechanism. Higher AN yields are, however, anticipated, up to a lofty upper limit of 82%, by allowing also for a consecutive mechanism (C3° → C =3 →AN). This possibility can be rationalized on the basis of the existence of vicinal C3° → C =3 /C =3 →AN sites whose presence is plentiful on the catalytically important ab planes of M1. The placement and efficiency of these sites is, however, not perfect; therefore the upper AN yield limit is not realized in practice. Our analysis of the elemental distribution at the active centers and their immediate surroundings provides us with new insights into the relationship between structure and catalytic reaction mechanisms of the M1 phase and might serve as a guide towards a redesign of the M1 composition, so as to attain higher AN yields from propane. It provides a challenging task for the synthetic chemist.















Similar content being viewed by others
References
Grasselli RK (2002) Top Catal 21:79
Grasselli RK, Tenhover MA (2008) Ammoxidation. In: Ertl G, Knözinger H, Schüth F, Weitkamp J (eds) Handbook of Heterogeneous Catalysis, 2nd ed, Ch. 14.11.9
Thomas JM (2011) Top Catal. This issue and references therein
Grasselli RK, Burrington JD, Buttrey DJ, DeSanto P Jr, Lugmair CG, Volpe AF Jr, Weingand T (2003) Top Catal 23:5
Hatano M, Kayo A (1988) European Patent 318 295
Ushikubo T, Oshima K, Kayo A, Umezawa T, Kiyono K, Sawaki I (1992) European Patent 529 853
Hinago H, Komada S, Kogyo AK (2000) US Patent 6,063,728
Hagemeyer A, Jandeleit B, Liu Y, Poojary DM, Turner HW, Volpe AF Jr, Weinberg WH (2001) Appl Catal A General 221:23
Borade R, Poojary D, Zhau X (2002) US Patent 6,395,552
Guan S, van Erden L, Haushalter H, Zhau X, Wang X, Srinivasen R (2002) EP 1,001,846 (2002); ibid., US Patent 6,149,882
Guram A, Hagemeyer A, Lugmair CG, Turner HW, Volpe AF Jr, Weinberg WH, Yaccato K (2004) Adv Synth Catal 346:215
Botella P, Conception P, Lopez-Nieto JM, Moreno Y (2005) Catal Today 99:51
Baca M, Aouine M, Dubois JL, Millet JMM (2005) J Catal 233:234
Grasselli RK, Buttrey DJ, Burrington JD, Andersson A, Holmberg J, Ueda W, Kubo J, Lugmair CG, Volpe AF Jr (2006) Top Catal 38:7
Grasselli RK (2005) Catal Today 99:23
Korochenko P, Shiju NR, Dozier AK, Graham UM, Guerro-Perez MO, Guliants VV (2008) Top Catal 50:43
Guliants VV (2011) Top Catal. This issue and references therein
Vaarkamp M, Ushikubo T (1998) Appl Catal A 174:99
Ushikubo T (2000) Catal Today 57:331
Ueda W, Vitry D, Katou T (2005) Catal Today 99:43
Grasselli RK, Buttrey DJ, DeSanto P Jr, Burrington JD, Lugmair CG, Volpe AF Jr, Weingand T (2004) Catal Today 91–92:251
Jang YH, Goddard WA III (2001) Top Catal 15:273
Grasselli RK (2011) Lett Catal. In preparation
Li X, Buttrey DJ, Blom DA, Vogt T (2011) Top Catal. This issue
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grasselli, R.K., Lugmair, C.G. & Volpe, A.F. Towards an Understanding of the Reaction Pathways in Propane Ammoxidation Based on the Distribution of Elements at the Active Centers of the M1 Phase of the MoV(Nb,Ta)TeO System. Top Catal 54, 595–604 (2011). https://doi.org/10.1007/s11244-011-9681-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-011-9681-2