Abstract
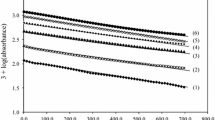
The kinetics and mechanism of permanganate oxidation of pyruvic acid in aqueous perchlorate at a constant ionic strength of 2.0 mol dm−3 have been investigated spectrophotometrically. The pseudo-first-order plots showed two distinct phases for the reaction, characterized as induction and auto-acceleration periods. The induction phase is relatively slow at the early stages of reaction and is followed by a faster process. The rate law for the oxidation was described by the sum of two exponential terms. The oxidation showed overall second-order kinetics with respect to the reactants in the initial slow phase. However, second-order kinetics was not maintained throughout the faster second stage. The effects of added salts lead to the conclusion that Mn(III) and/or Mn(IV) play the main role in the reaction kinetics of the second stage. A reaction mechanism consistent with the kinetic results is suggested and discussed.
Graphical Abstract








Similar content being viewed by others
References
Maleki N, Eiteman MA (2017) Fermentation 3:8
Zhang Y, Yao F, Du M, Ma C, Qiu J, Gu L, He X, Xu P (2010) Appl Microbiol Biotechnol 86:481
Rosche B, Sandford V, Breuer M, Hauer B, Rogers P (2001) Appl Microbiol Biotechnol 57:309
Reisse S, Haack M, Garbe D, Sommer B, Steffler F, Carsten J, Bohnen F, Sieber V, Bruck T (2016) Front Bioeng Biotechnol 4:74
Stine A, Zhang M, Ro S, Clendennen S, Shelton MC, Tyo KEJ, Broadbelt LJ (2016) Biotechnol Prog 32:303
Mazzio E, Soliman KF (2003) Neurochem Res 28:733
Wang X, Perez E, Liu R, Yan J-L, Mallet S-H (2016) Yang Brain Res 1132:1
Yoo MH, Lee JY, Lee SE, Hok JY, Yoon YH (2004) Invest Ophthalnol Visual Sci 45:1523
Borron ESG (1952) Trends in physiology and biochemistry. Academic Press, New York, p 471
Drummond AY, Waters WA (1955) J Chem Soc 497
Littler JS, Phil D, Thesis University of Oxford (1960)
Sen Gupta KK, Chatterjee HR (1978) Inorg Chem 17:2429
Sen Gupta KK, Sarkar T (1975) Tetrahedron 31:123
Albory WJ, Bell RP, Powel AL (1965) Trans Farad Soc 61:1194
Miklós J, Ivan VK, Eugen H (1992) Int J Chem Kin 24:1055
Hassan RM, Mousa MA,Wahdan MH (1988) J Chem Soc Dalton Trans 605
Hassan RM (1991) Cand J Chem 69:2018
Feigi F (1956) Spot test in organic analysis, vol 331. Elsevier, London, p 342
Hicks KW, Hurless MA (1983) Inorg Chim Acta 74:229
Hassan RM, Wahdan MH, Hassan A (1988) Eurp Poly J 24:281
Waters WA (1958) Q Rev Chem Soc 12:277–300
Radhakrishnamuti PS, Rao MD (1977) Ind J Chem Soc A 15:524
Girgis MM, Hassan RM, El-Shahawy AS (1987) Bull Fac Sci Assiut Univ 16:41–47
Nath P, Banerji KK, Bakore GV (1970) Bull Chem Soc JP 43:2027
Laidler K (1965) Chemical Kinetics. McGraw-Hill, New York
Cornforth JW, Popjak G (1949) Nature 164:1053
Curley K, Pratt RF (1997) J Org Chem 62:4479
Bally N, Carrington A, Lott KAK, Symons MCR (1960) J Chem Soc 290–297
Glasstone S, Laidler KJ, Eyring H (1941) Theory of rate processes. McGraw-Hill Book Company, New York, p 417
Millazzo GH, Caroll S, Sharma VK (1978) Tables of standard potentials. Wiley, New York
Latimer W (1952) Oxidation potentials, 2nd edn. Prentice-Hall, Englewood Cliffs
Marcus RA (1968) J Phys Chem 72:891
Hicks KW (1976) J Inorg Nucl Chem 38:1381
Hassan RM (2011) J Phys Chem 115:13338
Michaelis L, Menten ML (1913) Biochem Z 49:333
Girgis MM, El-Shatoury SA, Khalil ZH (1985) Cand J Chem 63:3317
Sen Gupta KK, Khamrui DK, Mukherjee DC (1975) Ind J Chem 13:348.34
Bakore GV, Shanker R (1963) Ind J Chem 1:108
Acknowledgements
This work was supported by Chemistry Department, Faculty of Science, Assiut University, Assiut 71516, Egypt. The authors would like to thank all staff members of Chemistry Department for their continuous encouragement throughout performing this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, R.M., Ibrahim, S.M. & Khairou, K.S. Kinetics and mechanism of oxidation of pyruvate by permanganate ion in aqueous perchlorate solution. Transit Met Chem 43, 683–691 (2018). https://doi.org/10.1007/s11243-018-0257-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0257-x