Abstract
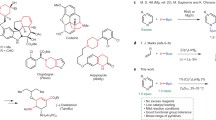
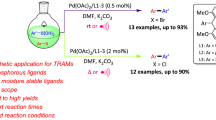
Catalytic routes for synthesis of alkyl benzoates by alkoxycarbonylation reactions and aryl benzoates by phenoxycarbonylation reactions of aryl iodides are described using a palladium–1,10-phenanthroline complex encaged in Y zeolite. Moderate to excellent yields (40–99 %) of various benzoates were obtained at low Pd loadings of 0.6 mol%. The catalyst could be effectively removed from the reaction mixture by a simple filtration process and was reused four times with only minor loss of activity. Furthermore, its catalytic activity was further highlighted by a comparison with another two supported Pd catalysts. The protocol has the advantages of easy handing, moderate to excellent yield, and catalyst recyclability.
Graphical Abstract



Similar content being viewed by others
References
Stetter J, Lieb F (2000) Angew Chem Int Ed 39:1724–1744
Larock RC (1989) Comprehensive organic transformations: a guide to functional group preparation. VCH, New York
Reddy RS, Rosa JN, Veiros LF, Caddick S, Gois PMP (2011) Org Biomol Chem 9:3126–3129
Colquhoun HM, Thompson DJ, Twigg MV (1991) Carbonylation: direct synthesis of carbonyl compounds. Plenum, NewYork
Mori M (2002) In: Negishi E (ed) Handbook of organopalladium chemistry for organic synthesis. Wiley, New York
Wu XF, Neumann H, Beller M (2013) Chem Rev 113:1–35
Brennführer A, Neumann H, Beller M (2009) Angew Chem Int Ed 48:4114–4133
Grigg R, Mutton SP (2010) Tetrahedron 66:5515–5548
Barnard CFJ (2008) Organometallics 27:5402–5422
Zapf A, Beller M (2005) Chem Commun 431–440. http://pubs.rsc.org/en/Content/ArticleLanding/2005/CC/B410937F#!divAbstract
Martinelli JR, Watson DA, Freckmann DMM, Barder TE, Buchwald SL (2008) J Org Chem 73:7102–7107
Liu JH, Chen J, Xia CG (2008) J Catal 253:50–56
Salvadori J, Balducci E, Zaza S, Petricci E, Taddei M (2010) J Org Chem 75:1841–1847
Antebi S, Arya P, Manzer LE, Alper H (2002) J Org Chem 67:6623–6631
Hao WY, Sha JC, Sheng SR, Cai MZ (2008) Catal Commun 10:257–260
Ullah E, McNulty J, Sliwinski M, Robertson A (2012) Tetrahedron Lett 53:3990–3993
Prasad AS, Satyanarayana B (2013) J Mol Catal A Chem 370:205–209
Khedkar MV, Sasaki T, Bhanage BM (2013) ACS Catal 3:287–293
Hu JL, Gu YL, Guan ZH, Li JJ, Mo WL, Li T, Li GX (2011) ChemSusChem 4:1767–1772
Aresta M, Giannoccaro P, Tommasi I, Dibenedetto A, Lanfredi AMM, Ugozzoli F (2000) Organometallics 19:3879–3889
Fan GZ, Zhang HJ, Cheng SQ, Ren ZD, Hu ZJ, Wang ZL (2008) Aust J Chem 61:610–614
Rostamnia S, Xin H (2013) Appl Organomet Chem 27:348–352
Ren N, Yang YH, Zhang YH, Wang QR, Tang Y (2007) J Catal 246:215–222
Xin Z, Gøgsig TM, Lindhardt AT, Skrydstrup T (2012) Org Lett 14:284–287
Zahmakiran M, Özkar S (2009) Langmuir 25:2667–2678
Beller M, Wu XF (2013) Transition metal catalyzed carbonylation reactions: carbonylation activation of C–X bonds. Springer, New York
Wu XF, Schranck J, Neumann H, Beller M (2011) ChemCatChem 4:69–71
Acknowledgments
This work is partly supported financially by the 863 Program of Ministry of Science and Technology of China (NC2010MA0137). Thankfulness is expressed for the spectroscopic analysis to the Analytical and Testing Center, Huazhong University of Science and Technology, Wuhan, China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mei, H., Xiao, S., Zhu, T. et al. Alkoxycarbonylation and phenoxycarbonylation reactions catalyzed by a palladium(II) organometallic complex encaged in Y zeolite. Transition Met Chem 39, 443–450 (2014). https://doi.org/10.1007/s11243-014-9818-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9818-9