Abstract
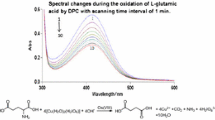
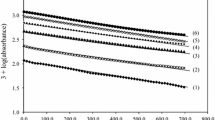
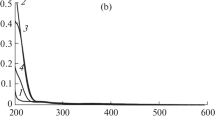
A potential synthetic biochromium source, bis-aspartatochromium(III) ion (where Asp is a tridentate N,O,O′-ligand, bonded via amine nitrogen and carboxylate oxygen atoms) has been obtained and characterized in aqueous solution. Kinetics of partial dechelation of the complex catalysed by H+ and OH− ions has been studied spectrophotometrically within 0.1–1.0 M HClO4 and 0.1–1.0 M NaOH ranges under first-order conditions. A linear dependence of the k obs,H on [H+] and independence of the k obs,OH on [OH−] were established. The derived rate expression and identification of components of the reaction mixture provide evidence for a reaction mechanism, where the key role in the overall process is the formation of an intermediate species with bidentate N,O-bonded Asp via both spontaneous and H+(OH−)-catalysed reaction paths. The intermediate is meta-stable and at pH 5–7 restores the substrate.












Similar content being viewed by others
References
Metzler DE (2001) Biochemistry, 2nd edn. Academic Press, Harcourt, p 53
Sekizaki M (1978) Bull Chem Soc Jpn 51:1991
Madafiglio K, Manning TM, Murdoch CM, Tulip WR, Cooper MK, Hambley TW, Freeman HC (1990) Acta Crystallogr Sect C Crystal Struct Commun 46:554
Viadhyanathan R, Bradshaw D, Rebilly J-N, BarrioJP, Gould JA, Berry NG, Rosseinsky (2006) Angew Chem Int Ed 45:6494
Barfod R, Bendahl L, Hammershoi A, Jensen DK, Sargeson AM, Willis AC (1999) J Chem Soc Dalton Trans 449–457
Atria AM, Valenzuela J, Vega A, Garcia AM (1997) J Chem Cryst 27:429
Okabe N, Mototsuji M, Yodoshi M (2007) Acta Crystallogr Sect E Struc Rep Online 6363:m2518
Marai H, Kita E, Kiersikowska E, Kuchta S, Bajek A, Drewa T (2012) Transit Met Chem 37:337
Kita E, Lisiak R (2011) Transit Met Chem 36:855
Marai H, Kita E, Wojciechowska J, Wróbel P (2012) Transit Met Chem 37:215
Kita E, Marai H, Muzioł T, Lenart L (2011) Transit Met Chem 36:35
Kiersikowska E, Marai H, Wrzeszcz G, Kita E (2013) Transit Met Chem 38:603
Pennington WT, Cordes AW, Kyle D, Wilson EW Jr (1984) Acta Crystallogr Sect C Cryst Struc Commun 40:1322
Bailey JL (1962) Techniques in protein chemistry. Elsevier, Amsterdam, p 73
Anderson RA, Polansky MM, Noella AB (2004) United States patent US 6,689,383 B1, Feb.10, 2004
Anderson RA (1998) J Am Coll Nutr 17:548
Slesinski RS, Clarke JJ, San RHC, Gudi R (2005) Mutation Res 585:86
Levina A, Codd R, Dillon CT, Lay PA(2003) Chromium in biology: toxicology and nutritional Aspects. In: Karlin KD (ed) Progress in inorganic chemistry, vol 51. Wiley, Hoboken, NJ, pp 145–250
Marai H, Kita E, Kuchta S (2012) Transit Met Chem 37:735
Buckingham DA, Clark CR, Miskelly GM (2000) Inorg Chem 39:61
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kita, E., Kiersikowska, E., Marai, H. et al. Kinetic studies on H+- and OH−-catalysed aquation of bis-aspartatochromium(III). Transition Met Chem 39, 63–70 (2014). https://doi.org/10.1007/s11243-013-9775-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9775-8