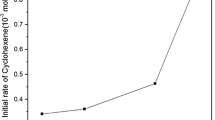
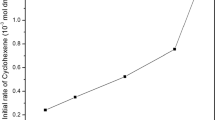
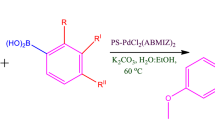
Abstract
A polymer anchored palladium complex was synthesized by sequential attachment of benzimidazole and palladium chloride to chloromethylated polystyrene divinyl benzene co-polymer with 6.5 % cross-linking. The product was characterized by XPS, UV–vis. spectrophotometry, FTIR and TGA. Various physico-chemical properties such as bulk density, surface area and swelling behavior in different solvents were also measured. The polymer anchored complex was tested as a catalyst for reduction of olefins. The kinetics of hydrogenation of 1-hexene was studied by varying the temperature, catalyst concentration and substrate concentration. The energy and entropy of activation were evaluated from the kinetic data. The catalyst could be recycled a number of times and no leaching of metal from the catalyst surface was observed.




Similar content being viewed by others
Abbreviations
- PSDVB:
-
Chloromethylated polystyrene divinyl benzene
- PSDVB-BzlH:
-
Benzimidazole Functionalized beads
- PSDVB-BzlH-PdCl2 :
-
Functionalized beads with PdCl2
References
Corma A, Serna P, Concepcio′n P, Calvino J (2008) J Am Chem Soc 130:8748–8753
Augustine RL (1997) Catal Today 37:419–440
Ramesh B, Sadanand DT, Reddy SG, Swamy KV, Saiprakash PK (2000) Trans Met Chem 25:639–643
Ertl G, Knozinger H, Schuth F, Weitkamp J (eds) (2008) Handbook of heterogeneous catalysis, vol 7, 2nd edn. Wiley-VCH, Verlag, GmbH & Co. KGaA, pp 3266–3329
Leadbeater NE, Marco M (2002) Chem Rev 102:3217–3274
Gupta KC, Sutar AK, Lin CC (2009) Coord Chem Rev 253:1926–1946
McNamara CA, Dixon MJ, Bradley M (2002) Chem Rev 102:3275–3300
Alexander S, Udayakumar V, Gayathri V (2009) J Mol Catal 314:21–27
Udayakumar V, Alexander S, Gayathri V, Shivakumaraiah B, Patil KR, Viswanathan B (2010) J Mol Catal 317:111–117
Armarego WLF, Chai CLL (2003) Purification of laboratory chemicals, 5th edn. Elsevier
Dalal MK, Ra RN (2001) Bull Mater Sci 24:237–241
Anthony R, Tembe GL, Ravindranathan M, Ram RN (2003) J Appl Polym Sci 90:370–378
Goodfellow RJ, Goggin PL, Venanzi LM (1967) J Chem Soc A11:1897–1900
Park PJD, Hendra PJ (1969) Spectrochim Acta 25A:909–916
Anderson C, Larsson R (1983) J Catal 81:179–193
Mani R, Mahadevan V, Srinivasan M (1990) Bri Poly J 22:177–184
Ferraro JR (1971) Low-frequency vibrations of inorganic and coordination compounds. Plenum Press, New York
Selvaraj PC, Mahadevan V (1997) J Polym Sci Polym Chem 35:105–122
Karklin LN, Iovel IG, Shimanskaya MV (1985) React Kinet Catal Lett 27:15–19
Bruner H, Bailar JC (1973) Inorg Chem 12:1465–1470
Gupta KC, Abdulkadir HK, Chand S (2003) J Mol Catal A 202:253–268
Sherrington DC (1998) Chem Commun 21:2275–2286
Candlin JP, Oldhalm AR (1968) Disc Far Soc 46:60–71
Kaneda K, Terasawa M, Imanaka T, Teranishi S (1976) Chem Lett 5:995–998
Jardine FH, Osborn JA, Wilkinson G (1967) J Chem Soc (A) 1574–1578
Nakamura Y, Hirai H (1974) Chem Lett 3:645–650
Zhang K, Neckers DC (1983) J Polym Sci A 21:3115–3127
Efraty A, Feinstein I (1982) Inorg Chem 21:3115–3118
Studies on polymer anchored and water soluble complexes of rhodium(I) as hydrogenation catalysts, Ph.D thesis by V. R. Parameshwaran, Dept. of Chemistry, IIT Madras 1990
Dalal MK, Gokak DT, Patel DR, Ram RN (1998) Ind J Chem Tech 5:376–382
Dalal MK, Ram RN (2000) J Mol Catal 159:285–292
Acknowledgments
The authors would like to thank UGC for DRS programme, Prof. Sadashiva, Liquid Crystal Lab. RRI for CHN analysis and DST, Central Facility, Physics Department, I.I.Sc. Bangalore for recording far-IR spectra. Thermax India Ltd. for providing chloromethylated poly(styrene–divinylbenzene) beads.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alexander, S., Udayakumar, V. & Gayathri, V. Catalytic activity of a supported palladium–benzimidazole complex toward alkene hydrogenation. Transition Met Chem 37, 367–372 (2012). https://doi.org/10.1007/s11243-012-9597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9597-0