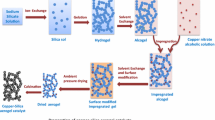
Abstract
Various aerogels of silica gel doped with Fe2O3 were prepared by sol–gel method. They were calcined to produce nanoparticle solids. The nanosized mixed oxides were active in the oxidation of alcohols and produced carbonyl compounds in very good to excellent yields using hydrogen peroxide.









Similar content being viewed by others
References
Cornell RM, Schwertmann U (1996) The iron oxides. VCH, Germany
Raj K, Moskovitz R (1990) J Magn Magn Mater 85:223
Levy L, Sahoo Y, Kim KS, Berrgey EJ, Prasad PN (2002) Chem Mater 14:3715–3721
Machala L, Zboril R, Gedank A (2007) J Phys Chem B 111:4003–4018
Nakamura T, Yamada Y, Yano K (2006) J Mater Chem 16:2417–2419
Ennas G, Musinu A, Piccaluga G, Zedda D, Gatteschi D, Sangregorio C, Stanger JL, Concas G, Spano G (1998) Chem Mater 10:495–502
Marchetti SG, Cagnoli MV, Alvarez AM, Gallegos NG, Bengoa JF, Yeramian AA, Mercader RC (1997) J Phys Chem Solids 58:2119–2125
Yi DK, Lee SS, Ying JY (2006) Chem Mater 18:2459–2461
Sato S, Takahashi R, Sodesawa T, Tanaka R (2003) Bull Chem Soc Jpn 76:217–223
Solzi M (1993) Fundamental properties of nanostructured materials. D. Fiorani and G. Sberveglieri, London
Khalil KMS, Makhlouf SA (2008) Appl Surf Sci 254:3767–3773
Dai Z, Meiser F, Möhwald H (2005) J Colloid Interface Sci 288:298–300
Tongpool R, Jindasuwan S (2005) Sens Actuators B 106:523–528
Fabrizioli P, Bürgi T, Baiker A (2002) J Catal 206:143–154
Clapsaddle BJ, Gash AE, Satcher JH Jr, Simpson RL (2003) J Non-Cryst Solids 331:190–201
Wang CT, Ro SH (2005) Appl Catal A 285:196–204
Savii C, Popovici M, Enache C, Subrt J, Niznansky D, Bakardzieva S, Caizer C, Hrianca I (2002) Solid State Ionics 151:219–227
Alcalá MD, Real C (2006) Solid State Ionics 177:955–960
Battishaa IK, Afify HH, Hamada IM (2005) J Magn Magn Mater 292:440–446
Bogatyrev VM, Guńko VM, Galaburda MV, Borysenko MV, Pokrovskiy VA, Oranska OI, Polshin EV, Korduban OM, Leboda R, Skubiszewska-Zieba J (2009) J Colloid Interface Sci 338:376–388
Fernández van Raap MB, Sanchez FH, Leyva AG, Japas ML, Cabanillas E, Troiani H (2007) Physica B 398:229–234
Martínez S, Meseguer M, Casas L, Rodríguez E, Molins E, Moreno-Maňas M, Roig A, Sebastián RM, Vallribera A (2003) Tetrahedron 59:1553–1556
Sheldon RA, Kochi JK (1981) Metal-catalyzed oxidation of organic compounds. Academic Press, New York
Hudlicky M (1990) Oxidations in organic chemistry. ACS, Washington, DC
Pybus DH, Sell CS (eds) (1999) The chemistry of fragrances. RSC Paperbacks, Cambridge
Fey T, Fischer H, Bachmann S, Albert K, Bolm C (2001) J Org Chem 66:8154–8159
Sheldon RA, Arends IWCE, Dijkman A (2000) Catal Today 57:157–166
Griffith WP, Joliffe JM (1991) Dioxygen activation and homogeneous catalytic oxidation. Elsevier, Amsterdam
Ley SV, Norman J, Griffith WP, Marsden SP (1994) Synthesis 25:639–666
Arends IWCE, Sheldon RA (2002) Top Catal 19:133–141
Warner PT, Warner JC (1998) Green chemistry theory and practice. Oxford University Press, New York
Cotton FA, Wilkinson G (1988) Advanced inorganic chemistry, 5th edn. Wiley, New York, p 458
Hosseini Monfared H, Amouei Z (2004) J Mol Catal A Chem 217:161–164
Hosseini-Monfared H, Ghorbani M (2001) Monatsh Chem 132:989–992
Hosseini-Monfared H, Ghadimi M (2003) J Chem Res S 313–314
Tatibouët JM (1997) Appl Catal A Gen 148:213–252
Wang CT, Willey RJ (2001) J Catal 202:211–219
Noyori R, Aoki M, Sato K (2003) Chem Commun 16:1977–1986
Cannas C, Gatteschi D, Musinu A, Piccaluga G, Sangregorio C (1998) J Phys Chem B 102:7721–7726
Kim GJ, Kim SH (1999) Catal Lett 57:139–143
Fabrizioli P, Bürgi T, Burgener M, Van Doorslaer S, Baiker A (2002) J Mater Chem 12:619–630
Armelao L, Granozzi G, Tondello E, Colombo P, Principi G, Lottici PP, Antonioli G (1995) J Non-Cryst Solids 192–193:435–438
Lippincott ER, Van Volkenburg A, Weir CE, Bunting EN (1958) J Res Natl Bur Stand 61:61–70
Cui H, Zayat M, Levy D (2009) J Alloys Compd 474:292–296
Cui H, Zayat M, Levy D (2005) Chem Mater 17:5562–5566
Niznansky D, Rehspringer JL (1995) J Non-Cryst Solids 180:191–196
Lane BS, Vogt M, DeRose VJ, Burgess K (2002) J Am Chem Soc 124:1946–1954
Ueno S, Yamaguchi K, Yoshida K, Ebitani K, Kaneda K (1998) Chem Commun 295–296
Huheey JE (1983) Inorganic chemistry: principles of structure and reactivity, 3rd edn. Harper and Row Publisher Inc, New York, p 340
Sheldon RA (1993) Top Curr Chem 164:21–34
Schumb WC, Satterfield CN, Wentworth RL (1955) Hydrogen peroxide. Reinhold, New York
Rode CV, Nehete UN, Dongare MK (2003) Catal Commun 4:365–369
Adam W, Rao PB, Degen HG, Saha-Mller CR (2000) J Am Chem Soc 122:5654–5655
Lu XH, Xia QH, Zhan HJ, Yuan HX, Ye CP, Su KX, Xu G (2006) J Mol Catal A Chem 250:62–69
Shul’pin GB, Golfeto CC, Süss-Fink G, Shul’pina LS, Mandelli D (2005) Tetrahedron Lett 46:4563–4567 and references therein
Pouralimardan O, Chamayou AC, Janiak C, Hosseini-Monfared H (2007) Inorg Chim Acta 360:1599–1608
Gross Z, Ini S (1997) J Org Chem 62:5514–5521
Collman JP, Brauman JI, Fitzgerald JP, Hampton PD, Naruta Y, Michida T (1988) Bull Chem Soc Jpn 61:47–57
Battioni P, Renaud JP, Bartoli JF, Reina-Artiles M, Fort M, Mansuy D (1988) J Am Chem Soc 110:8462–8470
Pillai UR, Sahle-Demessie E (2004) Appl Catal A 276:139–144
Wang J, Yan L, Li G, Wang X, Ding Y, Suo J (2005) Tetrahedron Lett 46:7023–7027
Wang X, Wu G, Wei W, Sun Y (2010) Transition Met Chem 35:213–220
Kumar V, Thankachan BPP, Prasad R (2010) Appl Catal A 381:8–17
Chaudhari MP, Sawant SB (2005) Chem Eng J 106:111–118
Jia A, Lou LL, Zhang C, Zhang Y, Liu S (2009) J Mol Catal A Chem 306:123–129
Salavati-Niasari M, Bazarganipour M (2009) Transition Met Chem 34:605–612
Xavier KO, Chacko J, Mohammed Yusuff KK (2004) Appl Catal A 258:251–259
Kanjina W, Trakarnpruk W (2009) Silpakorn U Science & Tech J 3:7–12
Shi F, Tse MK, Pohl MM, Radnik J, Brückner A, Zhang S, Beller M (2008) J Mol Catal A Chem 292:28–35
Neumann R, Levin-Elad M (1995) Appl Catal A 122:85–97
Acknowledgments
The authors thank the Zanjan University for the financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masoudian, S., Hosseini Monfared, H. & Aghaei, A. Silica aerogel–iron oxide nanocomposites: recoverable catalysts for the oxidation of alcohols with hydrogen peroxide. Transition Met Chem 36, 521–530 (2011). https://doi.org/10.1007/s11243-011-9498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-011-9498-7