Abstract
Three nickel(II) complexes, namely [Ni(BH)3](H2O)(NO3)(ClO4) 1, [Ni(BH)2(NO3)2] 2 and [Ni(BH)(Tren)](ClO4)2 3 (BH = Benzoylhydrazine, Tren = Tris(2-aminoethyl)amine) have been synthesized and characterized by physico-chemical techniques. X-ray crystallographic analysis shows the nickel to be six-coordinated in these complexes. The complexes are efficient catalysts for the dismutation of superoxide in alkaline DMSO-NBT assays. The IC50 values are 74,108 and 105 μM for 1, 2 and 3, respectively.
Graphical Abstract
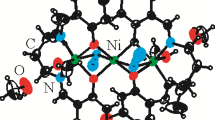
Three nickel(II) complexes with bidentate ligand viz.; [Ni(BH)3](NO3)(ClO4)(H2O) 1, [Ni(BH)2(NO3)2] 2 and [Ni(BH)(Tren)](ClO4)2 3 have been synthesized and characterized, including crystal structure, (Fig. 1) spectral characterization and electrochemical studies. X-ray crystallographic analysis shows the nickel to be six-coordinated mode in the respective complexes. The molecules of complexes 1 and 2 may stabilize in the crystal lattice due to the presence of π, π-interactions.







Similar content being viewed by others
References
Ainscough EW, Brodie AM, Ranford JD, Waters JM (1995) Inorg Chim Acta 236:83
Kocyigit KB, Rollas S (2002) II Farmaco 57:595
Stroupe ME, DiDonato M, Tainer JA (2001) In: Messer Schmidt A, Huber R, Poulos R, Weighardt K (eds) Handbook of metalloproteins. Wiley, England
Chaudhary SB (1999) Biochemistry 38:3744
Barondeaue DP (2004) Biochemistry 43:8038
Youn HD, Kin EJ, Roe JH, Hah YC, Kang SO (1996) J Biochem 318:889
McCord JM (2002) Superoxide Dismutase 349:331
Miller AF (2004) Cur Opin Chem Biol 8:162
Sheldrick GM (2008) Acta Crystallogr, Sect. A, A64, 112–122
Sheldrick GM (1997) Program for crystal structure solution and refinement. University of Goettingen, Goettingen
Patel RN, Singh N, Shukla KK, Chouhan UK, Niclós-Gutiérrez J, Castiñeiras A (2004) Inorg Chim Acta 357:2469
Patel RN, Singh N, Shukla KK, Chouhan UK, Niclós-Gutiérrez J, Castiñeiras A (2004) J Inorg Biochem 95:231
Bhirud RG, Shrivastava TS (1991) Inorg Chim Acta 179:125
Liu D, Kwasniewska K (1981) Bull Environ Contan Toxicol 27:289
Ya D, Svanidze OP, Dolgashova NV, Gogorishvili PV (1974) Zhurnal Neorganicheskoi Khimii 19(12):3304
Proinov I, Schwartz I, Ionescu C, Iliescu T (1967) Farmacia (Bucharest, Romania) 15(2):83
Johnson CK (1976) ORTEP, III Report ORNL- 5138. Oak Ridge National Laboratory, Oak, Ridge TN
Morelock MM, Lood M, Karrkan D, Maleki L, Eichet-Kerger HR, Magesti DJ (1999) J Am Chem Soc 101:4858
Geary WJ (1971) Coord Chem Rev 78:81
Le XY, Tang ML (2002) J Inorg Chem 18:1023
Jena S, Nath RN, Dash KC (1999) Indian J Chem 38A:350
Athar F, Arjmand F, Tabassum S (2001) Transition Met Chem 26:426
Johnson DK, Murphy TB, Rose NJ, Goodwin WH, Pickart L (1982) Inorg Chim Acta 67:159
Nakamoto K (1970) Infrared and Raman spectra of inorganic and coordination compounds, 2nd edn. Wiley-Interscience, New York
Karayannis MM, Mikulski CM, Pytlewski LL, Labes MM (1974) Inorg Chem 13:1146
Vansant C, Desseyn HO, Perlepes SP (1995) Transition Met Chem 20:454
Nakamoto K (1986) Infrared and Raman spectra of inorganic and coordination compounds, 4th edn. Wiley, New York
Lever ABP, Mantovaniand E, Ramaswamy BS (1971) Can J Chem 49:1957
Chikate RC, Padhye SB (2005) Polyhedron 24:1689
Kumar A, Kulkarni D, Patil SA, Badami PS (2009) J Sulf Chem 30:145
Parveen S, Arjmand F (2005) Indian J Chem 44A:1151
Kulkarni A, Avaji PJ, Bagihalli GB, Patil SA, Badami PS (2009) J Coord Chem 62:481
Bagihalli GB, Avaji PJ, Patil SA, Badami PS (2008) J Coord Chem 43:2639
Chohan ZH, Suphran CT, Scozzafava A (2004) J Enzyme Inhib Med Chem 79
Patel RN, Gundla VLN, Patel DK (2008) Polyhedron 27:1054
Frieden E (1975) J Chem Educ 52:754
Liao Z-R, Zheng X-F, Luo B-S, Shen L-R, Li D-F, Liu H-L, Zhao W (2001) Polyhedron 20:2813
Acknowledgments
Our grateful thanks are due to the National Diffraction Facility, X-ray Division, and RSIC (SAIF), IIT, Mumbai, for single crystal data collection. The Head RSIC (SAIF), Central Drug Research Institute, Lucknow, is also thankfully acknowledged for providing analytical and spectral facilities. Financial assistance from UGC [Scheme no. 36-28/2008 (SR) and DRDO] [Scheme no. ERIP/Er 0603574/m/01/1118], Delhi, is also thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A. Supplementary material
Appendix A. Supplementary material
CCDC 766594, 766595 and 766596 contain the supplementary crystallographic data for [Ni(BH)3](NO3)(ClO4)(H2O) 1, [Ni(BH)2(NO3)2] 2 and [Ni(BH)(Tren)](ClO4)2 3 (BH = Benzoylhydrazine, Tren = Tris(2-aminoethyl)amine). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Patel, R.N., Singh, A., Shukla, K.K. et al. Synthesis, characterization and biological activity studies of octahedral nickel(II) complexes. Transition Met Chem 36, 179–187 (2011). https://doi.org/10.1007/s11243-010-9451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-010-9451-1