Abstract
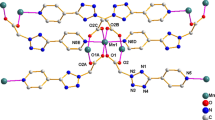
Three new mixed ligand complexes [Mn(4-pytone)2(bipy)2]bipy (1), [Mn(pot)2(en)2] (2) and [Mn(4-mot)2(en)2] (3) (4-pytone = 5-(4-pyridyl)-1,3,4-oxadiazole-2-thione, pot = 5-phenyl-1,3,4-oxadiazole-2-thione, 4-mot = 5-(4-methoxy-phenyl)-1,3,4-oxadiazole-2-thione) have been prepared containing bipy/en as coligands. The starting material potassium N-(aryl-carbonyl)-hydrazinecarbodithioates (RCONHNHCSSK) underwent cyclization during complexation in the presence of bipy or en to give the corresponding 5-aryl-1,3,4-oxadiazole-2-thiones. The complexes have been characterized by physicochemical techniques and single crystal X-ray structure determination. In all cases, the manganese has a six coordinate octahedral arrangement coordinated by 4N atoms of two bipy/en and two covalently bonded N atoms of the oxadiazole-2-thione anions.






Similar content being viewed by others
References
Wieghardt K (1989) Angew Chem Int Ed 28:1153. doi:10.1002/anie.198911531
Greenwood NN, Earnshaw A (1997) Chemistry of the Elements, 2nd edn. Reed educational and Professional, Oxford
Jakubkiene V, Burbuliene MM, Mekuskiene G, Udrenaite E, Gaidelis P, Vainilavicius P (2003) Il Farmaco 58:323. doi:10.1016/S0014-827X(02)00022-8
Tripathi P, Pal A, Jancik V, Pandey AK, Singh J, Singh NK (2007) Polyhedron 26:2597. doi:10.1016/J.poly.2006.12.046
Singh NK, Bharty MK, Dulare R, Butcher RJ (2009) Polyhedron 28:2443. doi:10.1016/J.poly.2009.04.030
Singh M, Butcher RJ, Singh NK (2008) Polyhedron 27:3151. doi:10.1016/J.poly.2008.08.007
Amin OH, Al-Hayaly LJ, Al-Jibori SA, Al-Allaf TAK (2004) Polyhedron 23:2013. doi:10.1016/J.poly.2004.05.006
Zhang ZH, Tian YL, Guo YM (2007) Inorg Chim Acta 360:2783. doi:10.1016/J.ica.2006.11.020
Zhang ZH, Li CP, Tang GM, Tian YL, Guo YM (2008) Inorg Chem Commun 11:326. doi:10.1016/J.poly.2006.09.008
Wang YT, Tang GM (2007) Inorg Chem Commun 10:53. doi:10.1016/Jinoche.2006.09.010
Du M, Zhang ZH, Zhao XJ, Xu Q (2006) Inorg Chem 45:5785. doi:10.1021/ic060129v
Wang YT, Tang GM (2007) Inorg Chem Commun 10:53. doi:10.1016/Jinoche.2006.09.010
Xu HX, Ma JP, Huang RQ, Dong YB (2005) Acta Cryst E61:m2462. doi:10.1107/S1600536805034987
Wang YT, Tang GM, Qiang ZW (2007) Polyhedron 26:4542. doi:10.1016/J.poly.2007.06.026
Obi K, Kojima A, Fukuda H, Hirai K (1995) Bioorg Med Chem Lett 5:2777. doi:10.1016/0960-894X(95)00485-C
Mishra L, Said MK, Itokawa H, Takeya K (1995) Bioorg Med Chem 3:1241. doi:10.1016/0968-0896(95)00095-X
Amabilino DB, Stoddart JF (1995) Chem Rev 95:2725. doi:10.1021/cr00040a005
Claessens CG, Stoddart JF (1997) J Phys Org Chem 10:254. doi:10.1002/(SICI)1099-1395(199705)10:5<254:AID-POC875>3.0.CO;2-3
Hirsch KA, Wilson SR, Moore JS (1997) Chem Eur J 3:765. doi:10.1002/Chem.19970030517
Lee RH, Griswold G, Kleinberg H (1964) Inorg Chem 3:1278. doi:10.1021/ic50019a019
Singh NK, Butcher RJ, Tripathi P, Srivastava AK, Bharty MK (2007) Acta Cryst E63:o782. doi:10.1107/S1600536806052238
Nonius (1998) COLLECT Nonius BV, Delft. The Netherlands
Otwinowski Z, Minor W (1997) In: Carter CW, Sweet RM (eds) Methods in enzymology, macromolecular crystallography, part A, vol 276. Academic Press, London, p 307
Altomare A, Burla MC, Camalli M, Cascarano GL, Giacovazzo C, Guagliardi A, Moliterni AGG, Polidori G, Spagna R (1999) J Appl Cryst 32:115. doi:10.1107/S0021889898007717
Oxford Diffraction (2007) CrysAlis RED and CrysAlis CCD Versions 1.171.31.8. Oxford Diffraction Ltd Abingdon, Oxafordshire, England
Sheldrick GM (2008) Acta Cryst A64:112. doi:10.1107/S0108767307043930
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) Acta Crystallogr Sect B58:389. doi:10.1107/S0108768102003324
Farrugia LJJ (1997) Appl Crystallogr 30:565. doi:10.1107/S0021889897003117
Molina P, Tarraga A, Espinosa A (1988) Synthesis 9:690. doi:10.1055/s-1988-27672
Patricia GS, Javier GT, Miguel AM, Francisco JA, Teofilo R (2002) Inorg Chem 41:1345. doi:10.1021/ic015625s
Lever ABP (1984) Inorganic electronic spectroscopy, 2nd edn. Elsevier, Amsterdam
Feng X, Shi XG, Ruan F (2009) Z. Kristallogr. NCS 224:193 ISSN:1433-7266
Chen XM, Huang XY, Xu YJ, Zhu YJ (1995) J Chemical Crystallography 25:605. doi:10.1007/BF01667032
Usuki N, Yamada M, Ohba M, Okawa H (2001) J Solid State Chem 159:328. doi:10.1006/JSSC.2001.9165
Bensch W, Nather C, Schur M (1997) Chem Commun 18:1773. doi:10.1039/a702844j
Janiak C (2000) J Chem Soc Dalton Trans 3885. doi:10.1039/b003010o
Acknowledgments
Authors thank CSIR, New Delhi for financial support by grant No. 01 (2152)07/EMR-II.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, N.K., Bharty, M.K., Kushawaha, S.K. et al. Manganese(II) complexes of 5-(4-pyridyl), 5-phenyl and 5-(4-methoxy-phenyl)-1,3,4-oxadiazole-2-thione containing 2, 2′-bipyridyl/ethylenediamine: synthesis, spectral, and X-ray characterization. Transition Met Chem 35, 337–344 (2010). https://doi.org/10.1007/s11243-010-9332-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-010-9332-7