Abstract
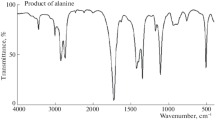
The kinetics of Ru(III) chloride-catalyzed oxidation of β-Alanine (NH3 +CH2CH2COOH, β-Ala) by N–bromophthalimide (NBP) in aqueous perchloric acid medium was studied at 35 °C. The rate law followed a first-order and zero-order dependence with respect to [NBP] and [β-Ala], respectively. The reaction followed first-order kinetics with respect to [Ru(III)] chloride at a range of low concentrations while the order changed from first- to zero-order at high concentration of [Ru(III)] chloride; demonstrating the catalytic effect for the oxidation of β-Ala by NBP. The rate decreased with increase in acidity. Chloride ions positively influenced the rate of the reaction. Neither phthalimide (NHP) nor Hg(II) influenced the reaction rate. Ionic strength (I) and dielectric constant (D) of the medium had no significant effect on the rate. Activation parameters of the reactions were determined by studying the reaction at different temperatures (30–50 °C). The colorimetric, FTIR, and GC-MS techniques were used to identify methyl cyanide (CH3CN) and CO2 as products of the reaction. In the reaction, approximately 2.3 moles of NBP oxidized one mole of β-Ala. A reaction scheme of the oxidation of β-Ala by NBP in the presence of Ru(III) chloride was found to be in consistent with the rate law and the reaction stoichiometry.






Similar content being viewed by others
References
Chandra G, Srivastava SN (1972) J Inorg Nucl Chem 34:197. doi:10.1016/0022-1902(72)80379-8
Rao VS, Sethuram B, Rao TN (1979) Int J Chem Kinet 11:165. doi:10.1002/kin.550110208
Kantouch A, Fattah SHA (1971) Chem Zvest 25:222
Singh AK, Chopra D, Rahmani S, Singh B (1998) Carbohydr Res 314:157. doi:10.1016/S0008-6215(98)00322-X
Singh AK, Singh V, Singh AK, Gupta N, Singh B (2002) Carbohydr Res 337:345. doi:10.1016/S0008-6215(01)00319-6
Singh AK, Singh V, Rahmani S, Singh AK, Singh B (2003) J Mol Cataly A: Chem 197:91
Singh AK, Srivastava J, Rahmani S, Singh V (2006) Carbohydr Res 341:397. doi:10.1016/j.carres.2005.11.012
Singh AK, Singh V, Ashish Srivastava J (2006) Ind J Chem 45A:599
Rangappa KS, Raghvendra MP, Mahadevappa DS, Gowda DC (1998) Carbohydr Res 306:56. doi:10.1016/S0008-6215(97)00243-7
Gowda BT, Damodara N, Jyothi K (2007) Int J Chem Kinet 37:572. doi:10.1002/kin.20103
Mukherjee N, Banerjee KK (1981) J Org Chem 46:2323. doi:10.1021/jo00324a022
Filler R (1963) Chem Rev 63:21. doi:10.1021/cr60221a002
Khanchandani R, Sharma PK, Banerji KK (1996) Ind J Chem 35A:57
Chaudhary K, Sharma PK, Banerji KK (1999) Int J Chem Kinet 31:469. doi:10.1002/(SICI)1097-4601(1999)31:7<469::AID-KIN1>3.0.CO;2-2
Vyas, Shashi, Sharma PK (2001) Oxdn Commun 24:248
Kumbhat V, Sharma PK, Banerjee KK (2002) Int J Chem Kinet 34:248. doi:10.1002/kin.10036
Thiagarajan V, Venkatasubramanian N (1970) Ind J Chem 8A:809
Bachhawat JN, Mathur NK (1971) Ind J Chem 9A:1335
Jabbar SFA, Surender RV (1994) Ind J Chem 33A:69
Singh B, Srivastava S (1991) Trans Met Chem 16:466. doi:10.1007/BF01129466
Jagdeesh RV, Puttaswamy (2008) J Phys Org Chem 21:844. doi:10.1002/poc.1379
Sharma JP, Singh RNP, Singh AK, Singh B (1986) Tetrahedron 42:2739. doi:10.1016/S0040-4020(01)90561-7
Singh AK, Srivastava J, Rahmani S (2007) J Mol Cataly A: Chem 271:151
Singh B, Singh AK, Singh D (1988) J Mol Cataly A: Chem 48:207
Huo S-Y, Song C-Y, Zhen Y-J (2007) Trans Met Chem 32:936. doi:10.1007/s11243-007-0257-8
Katre YR, Joshi GK, Singh AK (2008) Tenside Surf Det 45:213
Soloway S, Lipschietz A (1952) Anal Chem 24:898. doi:10.1021/ac60065a041
Singh AK, Rahmani S, Singh B, Singh RK, Singh M (2004) J Phys Org Chem 17:249. doi:10.1002/poc.723
Bailar JC (1956) The chemistry of co-ordination compounds. Reinhold, New York, p 14
Krishnakumar V, Balachandran V, Chithambarathanu T (2005) Spectrochim Acta 62:918. doi:10.1016/j.saa.2005.02.051
Khazaei A, Manesh AA (2005) J Braz Chem Soc 16:874. doi:10.1590/S0103-50532005000500030
Kirsch A, Luning U (1998) J Prakt Chem 340:129. doi:10.1002/prac.19983400205
Kirsch A, Luning U, Kruger O (1999) J Prakt Chem 341:649. doi:10.1002/(SICI)1521-3897(199910)341:7<649::AID-PRAC649>3.0.CO;2-L
Day JC, Govindaraj N, McBain DS, Skell PS, Tanko JM (1986) J Org Chem 51:4959. doi:10.1021/jo00375a038
Ramachandrappa R, Puttaswamy, Gowda NMM (1998) Int J Chem Kinet 30:407. doi:10.1002/(SICI)1097-4601(1998)30:6<407::AID-KIN2>3.0.CO;2-W
Das CM, Indrasenan P (1986) Ind J Chem 25A:605
Das CM, Indrasenan P (1987) Ind J Chem 26A:717
Das CM, Indrasenan P (1984) Ind J Chem 23A:869
Taqui Khan MM, Chandraiah GR, Rao AP (1986) Inorg Chem 25:665. doi:10.1021/ic00225a015
Acknowledgments
One of the authors (AKS) thank the University Grant Commission, Regional Office Bhopal, MP for Minor Research Project grant. Authors wish to thank the reviewer for greatly improving the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.K., Jain, B., Negi, R. et al. Kinetics and mechanism of oxidation of β-Alanine by N-bromophthalimide in the presence of Ru(III) chloride as homogenous catalyst in acidic medium. Transition Met Chem 34, 521–528 (2009). https://doi.org/10.1007/s11243-009-9225-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9225-9