Abstract
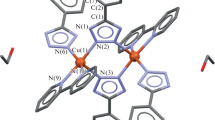
Three new Cu(II) complexes with ethyl bis(2-pyridylmethyl)amino-2-propionate (Etdpa), or bis(2-pyridylmethyl)amino-2-propionate (Adpa), were synthesized and characterized by physico-chemical and spectroscopic methods. The X-ray crystal structure of [(Adpa)CuCl] shows that the copper(II) atom is coordinated by three N atoms, one oxygen atom from the ligand (Adpa) and one chloride anion, forming a trigonal bipyramidal geometry. The spectrophotometric and fluorescence titration data indicate that the interaction of square pyramidal [(Etdpa)CuCl2] with ct-DNA is weak, but the trigonal bipyramidal complexes [(Adpa)Cu(H2O)](ClO4) and [(Adpa)CuCl] interact with ct-DNA with the mode of intercalation. The inhibition activities of the three new copper(II) complexes on the four cancer cells (Mcf-7, Eca-109, A549, and Hela) are in the order: [(Adpa)Cu(H2O)](ClO4) > [(Adpa)CuCl] > [(Etdpa)CuCl2], which correlates with their DNA-binding properties. The results show that the substituents introduced on the secondary amino nitrogen atom of dpa have great contribution to the antitumor activities of these copper(II) complexes. It is also found that the coordination of copper(II) ions with AdpaH can decrease the toxicity of AdpaH.






Similar content being viewed by others
References
Kumar CV, Barton JK, Turro MJ (1985) J Am Chem Soc 107:5518. doi:1.101021/ja00305a033
Solomon EI, Sundaram UM, Machonkin TE (1996) Chem Rev 96:2563. doi:10.1021/cr950046o
Lewis EA, Tolman WB (2004) Chem Rev 104:1047. doi:10.1021/cr020633r
Apelgot S, Coopy J, Fromentin A, Guille E, Poupon MF, Roussel A (1986) Anticancer Res 6:159
Hambley TW (2007) J Chem Soc Dalton Trans 43:4929. doi:10.1039/6706075k
Maiti D, Lucas HR, Sarjeant AAN, Karlin KD (2007) J Am Chem Soc 129:6998. doi:10.1021/ja071704c
Zhou CJ, Zhao J, Wu YB, Yin CX, Yang P (2007) J Inorg Biochem 101:10. doi:10.1016/j.jinorgbio.2006.07.011
Choi KY, Ryu H, Suny ND (2003) J Chem Crystallogr 32:947. doi:1074-1542/03/1200-0947
Kruppa M, Konig B (2006) Chem Rev 106:3520. doi:10.1021/cr010206y
Kalinowski DS, Yu Y, Sharpe PC, Islam M, Liao YT, Lovejoy DB, Kumar N, Bernhardt PV, Richardson DR (2007) J Med Chem 50:3716. doi:10.1021/jm0704452
Fernandes C, Parrilha GL, Lessa JA, Santiago LJM, Kanashiro MM, Boniolo FS, Bortoluzzi AJ, Vugman NV (2006) Inorg Chim Acta 359:3167. doi:10.1016/j.ica.2006.04.007
Sheldrick GM (1997) SHELXTL-97, program for crystal structure solution and refinement. University of Gottingen, Germany
Reichmann ME, Rice SA, Thomas CA, Doty PJ (1954) J Am Chem Soc 76:3047. doi:10.1021/ja01640a067
Barton JK, Danishefsky AT, Golderg JM (1984) J Am Chem Soc 106:2172. doi:10.1021/ja00319a043
Dewey TG (1991) Biophysical and biochemical aspects of fluorescence spectroscopy. Plenum, New York, pp 1–41
Lakowica JR, Weber G (1973) Biochemistry 12:4161. doi:10.1021/bi00745a020
Wang D, Narang A, Kotb M, Gaber DO, Miller DD, Kim SW, Mahoto RI (2002) Biomacromolecules 3:1197. doi:10.1021/bm025563c
Bakalbassis EG, Tsipis CA, Bozopoulos AP, Dreissig DW, Hartl H, Mrozinski J (1991) Inorg Chem 30:2801. doi:10.1021/ic00013a018
Choi KY, Jeon YM, Ryu H, Oh JJ, Lim HH, Kim MW (2004) Polyhedron 23:903. doi:10.1016/j.poly.2003.11.058
Ling KQ, Lawrence M (2005) J Am Chem Soc 127:4777. doi:10.1021/ja455603
Utz D, Kisslinger S, Hampel F, Schindle S (2008) J Inorg Biochem 102:1236. doi:10.1016/j.jinorgbio.2008.01.028
Karlin KD, Hapes JC, Juen S, Hutchinson JP, Iubieta J (1982) Inorg Chem 21:4106. doi:10.1021/ic00141a049
Hartman JR, Vachet RW, Pearson W, Wheat RJ, Callahan JH (2003) Inorg Chim Acta 343:119. doi:0020-1693/02/01229-x
Li QS, Yang P, Wang HF, Guo ML (1996) J Inorg Biochem 64:181. doi:0162-0134196
Hathaway BJ (1991) Struct Bonding 57:2801
Rao R, Patra AK, Chetana PR (2008) Polyhedron 27:1343. doi:10.1016/j.poly.2007.12.026
Boger DL, Fink BE, Brunette SR, Tse WC, Hedrick MP (2001) J Am Chem Soc 123:5878. doi:10.1021/ja010041a
Wang BD, Yang ZY, Wang Q (2006) Bioorgan Med Chem 14:1880
Biver T, Secco F, Tine MR, Venturini M (2004) J Inorg Biochem 98:33. doi:10.1016/j.jinorgbio.2003.08010
Ware WR (1962) J Phys Chem 66:455. doi:10.1021/j100809a020
Acknowledgements
Financial support from National Science Foundation of China (20777029B0702), distinguished scholar science foundation of Jiangsu University (06JDG050), and the foundation of state key Laboratory of Coordination Chemistry, Nanjing University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC No. 691109 for 3. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Wang, LY., Chen, QY., Huang, J. et al. Synthesis, characterization, and bioactivities of copper complexes with N-substituted Di(picolyl)amines. Transition Met Chem 34, 337–345 (2009). https://doi.org/10.1007/s11243-009-9200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9200-5