Abstract
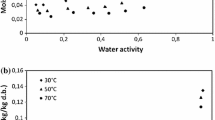
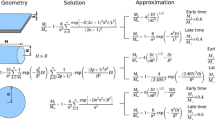
The kinetics and equilibrium of water vapor adsorption/desorption isotherm (WVSI) are fundamental information for solid–moisture interaction in the microstructure of cement-based porous materials (CBPM). This paper presents firstly the experimental data for WVSI of CBPM with a ternary binder. Mass changes in specimen are recorded in sorption tests for materials with different aggregate contents and water-to-binder (w/b) ratios under controlled ambient relative humidity. Both the sorption equilibrium and sorption kinetics are investigated through some established physical models, and the corresponding intrinsic parameters of moisture sorption are determined. For sorption kinetics, the mass change during sorption is interpreted through Elovich model, power model and pseudo-first-order and pseudo-second-order models. The mass change in sorption occurs mainly in the first 40 days, and the pseudo-second-order model shows the best adaption to all adsorption/desorption processes. For sorption equilibrium, Guggenheim–Anderson–de Boer (GAB), modified Halsey and Oswin models are used to interpret the moisture sorption capacity. The hysteresis between adsorption and desorption processes is attributed, respectively, to the mechanisms of snap-through, energy instability and pore constrictivity for low, middle and high relative humidity ranges. From the analysis, it is found that (1) through the interpretation of pseudo-second-order model the moisture sorption kinetics can be controlled by the sorption between the water molecules and the pore wall in addition to vapor diffusion; (2) the sorption capacity is sensitive to aggregate content and w/b ratio; incorporating aggregates, decreasing w/b ratio and increasing humidity gradient tend to decrease the sorption capacity of CBPMs; (3) the sorption isotherm hysteresis is well described by GAB-H model, both the extent of hysteresis and the energy constant related to pore surface tension showing clear correlation with the aggregate content and w/b ratio of CBPMs.















Similar content being viewed by others
References
Adolphs, J.: Surface energy of hardened cement paste depending on relative humidity. Mater. Struct. 38, 443–448 (2005)
Alford, N.M., Rahman, A.A.: An assessment of porosity and pore sizes in hardened cement pastes. J. Mater. Sci. 16, 3105–3114 (1981)
Aligizaki, K.K.: Pore structure of cement-based materials: testing, interpretation and requirement. Taylor and Francis, New York (2006)
Allen, A.J., Thomas, J.J., Jennings, H.M.: Composition and density of nanoscale calcium—silicate—hydrate in cement. Nat. Mater. 6(4), 311–316 (2007)
Baroghel-Bouny, V.: Water vapour sorption experiments on hardened cementitious materials Part I: essential tool for analysis of hygral behaviour and its relation to pore structure. Cem. Concr. Res. 37, 414–437 (2007a)
Baroghel-Bouny, V.: Water vapour sorption experiments on hardened cementitious materials Part I: essential tool for assessment of transport properties and for durability prediction. Cem. Concr. Res. 37, 438–454 (2007b)
Bashiri, H.: Desorption kinetics at the solid/solution interface: a theoretical description by statistical rate theory for close-to-equilibrium systems. J. Phys. Chem. C. 115, 5732–5739 (2011)
Bazant, Z.P.: Thermodynamics of interacting continua with surfaces and creep analysis of concrete structures. Nucl. Eng. Des. 20, 477–505 (1972a)
Bazant, Z.P.: Thermodynamics of hindered adsorption with application to cement paste and concrete. Cem. Concr. Res. 2, 1–16 (1972b)
Bazant, Z.P., Hauggaard, A.B., Baweja, S., Ulm, F.-J.: Microprestress-solidification theory for concrete creep. I: aging and drying effects. J. Eng. Mech. 123, 1188–1194 (1997)
Bazant, M.Z., Bazant, Z.P.: Theory of sorption hysteresis in nanoporous solids: Part I snap-through instabilities. J. Mech. Phys. Solids 60, 1644–1659 (2012a)
Bazant, M.Z., Bazant, Z.P.: Theory of sorption hysteresis in nanoporous solids: Part II molecular condensation. J. Mech. Phys. Solids 60, 1660–1675 (2012b)
Benzarti, K., Perruchot, C., Chehimi, M.M.: Surface energetics of cementitious materials and their wettability by an epoxy adhesive. Coll. Surf. A 286, 78–91 (2006)
Bizot, H.: Using the GAB model to construct sorption isotherms. In: Jowitt, R., et al. (eds.) Physical Properties of Foods (European Project Group COST 90 on physical properties of foods). Applied Science Publishers, London (1983)
Bratasz, L., Kozlowska, A., Kozlowski, R.: Analysis of water adsorption by wood using the Guggenheim–Anderson–de Boer equation. Eur. J. Wood Prod. 70, 445–451 (2012)
Brennan, J.K., Thomson, K.T., Gubbins, K.E.: Adsorption of water in activated carbons: effects of pore blocking and connectivity. Langmuir 18, 5438–5447 (2002)
Brue, F., Davy, C.A., Skoczylas, F., Burlion, N., Bourbon, X.: Effect of temperature on the water retention properties of two high performance concretes. Cem. Concr. Res. 42, 384–396 (2012)
Carlier, JPh, Rougelot, Th, Burlion, N.: Performance evaluation of models describing sorption isotherm in cementitious materials between saturation and oven dryness. Constr. Build. Mater. 37, 58–66 (2012)
Derluyn, H., Derome, D., Carmeliet, J., Stora, E., Barbarulo, R.: Hysteretic moisture behavior of concrete: modeling and analysis. Cem. Concr. Res. 42(10), 1379–1388 (2012)
Dutcher, C.S., Ge, X., Wexler, A.S., Clegg, S.L.: Statistical mechanics of multilayer sorption: extension of the Brunauer–Emmett–Teller (BET) and Guggenheim–Anderson–de Boer (GAB) adsorption isotherms. J. Phys. Chem. C 115(33), 16474–16487 (2011)
Espinosa, R.M., Franke, L.: Influence of the age and drying process on pore structure and sorption isotherms of hardened cement paste. Cem. Concr. Res. 36, 1969–1984 (2006)
Febrianto, J., Kosasih, A.N., Sunarso, J., Ju, Y.H., Indraswati, N., Ismadji, S.: Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J. Hazard. Mater. 162, 616–645 (2009)
Furmaniak, S., Gauden, P.A., Terzyk, A.P., Rychlicki, G.: Water adsorption on carbons: critical review of the most popular analytical approaches. Adv. Colloid Interface 137, 82–143 (2008)
Garbalinska, H., Kowalski, S.J., Staszak, M.: Moisture transfer between unsaturated cement mortar and ambient air. Transp. Porous Med. 85(1), 79–96 (2010)
Garbalinska, H., Kowalski, S.J., Staszak, M.: Moisture diffusivity in mortars of different water-cement ratios and in narrow ranges of air humidity changes. Int. J. Heat Mass Transf. 56, 212–222 (2013)
Hagymassy, J.J.R., Brunauer, S., Mikhail, R.S.: Pore structure analysis by water vapour adsorption: t-curves for water vapour. J. Colloid Interface Sci. 29(3), 485–491 (1969)
Halsey, G.: Physical adsorption on non-uniform surfaces. J. Chem. Phys. 16, 931–937 (1948)
Ho, Y.S.: Review of second-order models for adsorption systems. J. Hazard. Mater. B136, 681–689 (2006)
Horikawa, T., Do, D.D., Nicholson, D.: Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface 169, 40–58 (2011)
Horikawa, T., Sakao, N., Do, D.D.: Effects of temperature on water adsorption on controlled microporous and mesoporous carbonaceous solids. Carbon 56, 183–192 (2013)
Janczuk, B., Biakopiotrowicz, T.: Components of surface free energy of some clay minerals. Clays Clay Miner. 36, 243–248 (1988)
Jennings, H.M.: Refinements to colloid model of C–S–H in cement: CM-II. Cem. Concr. Res. 38, 275–289 (2008)
Jiang, J., Yuan, Y.: Relationship of moisture content with temperature and relative humidity in concrete. Mag. Concr. Res. 65(11), 685–692 (2013)
Lesti, M., Tiemeyer, C., Plank, J.: \(\text{ CO }_{2}\) stability of Portland cement based well cementing systems for use on carbon capture and storage (CCS) wells. Cem. Concr. Res. 45, 45–54 (2013)
Li, H., Ai, M., Liu, B., Zheng, S., Zong, G.: Water vapor sorption on surfactant-templated porous silica xerogels. Microporous Mesoporous Mater. 143(1), 1–5 (2011)
Li, H.G., Huang, G.H., An, C.J., Zhang, W.X.: Kinetic and equilibrium studies on the adsorption of calcium lignosulfonate from aqueous solution by coal fly ash. Chem. Eng. J. 200–202, 275–282 (2012)
López-Aranguren, P., Saurina, J., Vega, L.F., Domingo, C.: Sorption of tryalkoxysilane in low-cost porous silicates using a supercritical \(\text{ CO }_{2}\) method. Microporous Mesoporous Mater. 148(1), 15–24 (2012)
Lyn, M.E., Burnett, D., Garcia, A.R., Gray, R.: Interaction of water with three granular biopesticide formulations. J. Agric. Food Chem. 58, 1804–1814 (2010)
McLintock, J.S.: The Elovich equation in chemisorption kinetics. Nature 216, 1205–1205 (1967)
Mehta, P.K., Monterio, P.J.M.: Concrete, Microstructure, Properties and Materials. McGraw-Hill, London (2006)
Miah, M.Y., Volchek, K., Kuang, W.X., Tezel, F.H.: Kinetic and equilibrium studies of cesium adsorption on ceiling tiles from aqueous solutions. J. Hazard. Mater. 183, 712–717 (2010)
Moradi, O., Fakhri, A., Adami, S., Adami, S.: Isotherm, thermodynamic, kinetics, and adsorption mechanism studies of Ethidium bromide by single-walled carbon nanotube and carboxylate group functionalized single-walled carbon nanotube. J. Colloid Interface Sci. 395, 224–229 (2013)
Mugge, J., Bosch, H., Reith, T.: Measuring and modelling gas adsorption kinetics in single porous particles. Chem. Eng. Sci. 56, 5351–5360 (2001)
Oh, J.S., Shim, W.G., Lee, J.W., Kim, J.H., Moon, H., Seo, G.: Adsorption equilibrium of water vapor on mesoporous materials. J. Chem. Eng. Data 48, 1458–1462 (2003)
Oswin, C.R.: The kinetics of package life. III. Isotherm. J. Soc. Chem. Ind. 65(12), 419–421 (1946)
Pavlik, Z., Zumar, J., Medved, I., Cerny, R.: Water vapor adsorption in porous building materials: experimental measurement and theoretical analysis. Transp. Porous Med. 91(3), 939–954 (2012)
Pellenq, R.J.-M., Coasne, B., Denoyel, R.O., Coussy, O.: Simple phenomenological model for phase transitions in confined geometry. 2. Capillary condensation/evaporation in cylindrical mesopores. Langmuir 25, 1393–1402 (2009)
Petrov, O., Furo, I.: Curvature-dependent metastability of the solid phase and the freezing-melting hysteresis in pores. Phys. Rev. E. 73, 0110608(7p) (2006)
Plazinski, W., Rudzinski, W., Plazinska, A.: Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv. Colloid Interface 152, 2–13 (2009)
Plazinski, W., Dziuba, J., Rudzinski, W.: Modeling of sorption kinetics: the pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 19(5), 1055–1064 (2013)
Powers, T.C.: Some observations on the interpretation of creep data. Bull. RILEM (Paris) 1966(33), 381–391 (1966)
Poyet, S.: Experimental investigation of the effect of temperature on the first desorption isotherm of concrete. Cem. Concr. Res. 39, 1052–1059 (2009)
Poyet, S., Charles, S., Honoré, N., L’hostis, V.: Assessment of the unsaturated water transport properties of an old concrete: determination of the pore-interaction factor. Cem. Concr. Res. 41, 1015–1023 (2011)
Poyet, S.: Determination of the intrinsic permeability to water of cementitious materials: influence of the water retention curve. Cem. Concr. Compos. 35(1), 127–135 (2013)
Pradas, M.M., Sánchez, M.S., Ferrer, G.G., Ribelles, J.M.G.: Thermodynamics and statistical mechanics of multilayer adsorption. J. Chem. Phys. 121(17), 8524–8531 (2004)
Qin, M., Belarbi, R., Ait-Mokhtar, A., Nilsson, L.O.: Nonisothermal moisture transport in hygroscopic building materials: modeling for the determination of moisture transport coefficients. Transp. Porous Med. 72(2), 255–271 (2008)
Ranaivomanana, H., Verdier, J., Sellier, A., Bourbon, X.: Toward a better comprehension and modeling of hysteresis cycles in the water sorption-desorption process for cement based materials. Cem. Concr. Res. 41, 817–827 (2011)
Richardson, I.G.: Tobermorite/jennite-and tobermorite/calcium hydroxide-based models for the structure of C–S–H: applicability to hardened pastes of tricalcium silicate, \(\beta \)-dicalcium silicate, Portland cement, and blends of Portland cement with blast-furnace slag, metakaolin, or silica fume. Cem. Concr. Res. 34, 1733–1777 (2004)
Saeidpour, M., Wadsö, L.: Moisture equilibrium of cement based materials containing slag or silica fume and exposed to repeated sorption cycles. Cem. Concr. Res. 69, 88–95 (2015)
Scrivener, K.L., Crumbie, A.K., Laugesen, P.: The interfacial transition zone (ITZ) between cement paste and aggregate in concrete. Interface Sci. 12, 411–421 (2004)
Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pieotti, R.A., Rouquerol, J., Siemieniewska, T.: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57(4), 603–619 (1985)
Tennis, P.D., Jennings, H.M.: A model for two types of calcium silicate hydrate in the microstructure of Portland cement pastes. Cem. Concr. Res. 30, 855–863 (2000)
Thiery, M., Dangla, P., Belin, P., Habert, G., Roussel, N.: Carbonation kinetics of a bed of recycled concrete aggregates: a laboratory study on model materials. Cem. Concr. Res. 46, 50–65 (2013)
Thomas, J.J., Jennings, H.M.: A colloidal interpretation of chemical aging of the C–S–H gel and its effects on the properties of cement paste. Cem. Concr. Res. 36, 30–38 (2006)
Timmermann, E.O.: Multilayer sorption parameters: BET or GAB values? Colloid Surf. A 220, 235–260 (2003)
Volchek, K., Miah, M.Y., Kuang, W.X., DeMaleki, Z., Tezel, F.H.: Adsorption of cesium on cement mortar from aqueous solutions. J. Hazard. Mater. 194, 331–337 (2011)
Wang, Q., Yan, P., Mi, G.: Effect of blended steel slag–GBFS mineral admixture on hydration and strength of cement. Constr. Build. Mater. 35, 8–14 (2012)
Wu, M., Johannesson, B., Geiker, M.: A study of the water vapor sorption isotherms of hardened cement pastes: possible pore structure changes at low relative humidity and the impact of temperature on isotherms. Cem. Concr. Res. 56, 97–105 (2014)
Yang, W., Sokhansanj, S., Cenkowski, S., Tang, J., Wu, Y.: A general model for sorption hysteresis in food materials. J. Food Eng. 33, 421–444 (1997)
Zeng, Q., Li, K.: Reaction and microstructure of cement-fly-ash system. Mater. Struct. 48, 1703–1716 (2015)
Zeng, Q., Li, K., Fen-Chong, T., Dangla, P.: Pore structure characterization of cement pastes blended with high-volume fly-ash. Cem. Concr. Res. 42, 194–204 (2012a)
Zeng, Q., Li, K., Fen-Chong, T., Dangla, P.: Determination of cement hydration and pozzolanic reaction extents for fly-ash cement pastes. Constr. Build. Mater. 27, 560–569 (2012b)
Zeng, Q., Li, K., Fen-Chong, T., Dangla, P.: Analysis of pore structure, contact angle and pore entrapment of blended cement pastes from mercury porosimetry data. Cem. Concr. Compos. 34, 1053–1060 (2012c)
Zeng, Q., Li, K., Fen-Chong, T., Dangla, P.: Water removal by freeze-drying of hardened cement paste. Dry. Technol. 31, 67–71 (2013)
Zhang, Z., Thiery, M., Baroghel-Bouny, V.: A review and statistical study of existing hysteresis models for cementitious materials. Cem. Concr. Res. 57, 44–60 (2014)
Zhang, J., Scherer, G.W.: Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 41, 1024–1036 (2011)
Acknowledgments
The research is supported by NSFC Project Grant No. 51378295. Also, the help of Mr. Zhiling Zhang and Mr. Yuyang Wang is acknowledged for their work on the sorption experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, Q., Zhang, D. & Li, K. Kinetics and Equilibrium Isotherms of Water Vapor Adsorption/Desorption in Cement-Based Porous Materials. Transp Porous Med 109, 469–493 (2015). https://doi.org/10.1007/s11242-015-0531-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-015-0531-8