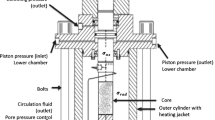
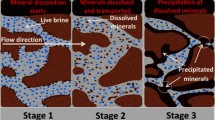
Abstract
Effect of the aqueous chemistry on the mechanical strength of chalk has extensively been studied during the last decade. At high temperatures (~130°C), chalk exposed to seawater is significantly weaker compared to chalk exposed to distilled water when considering the hydrostatic yield strength and the following creep phase. The explanation of these experimental results must be of a chemical nature, as the density and viscosity of the aqueous phase vary little among these different brines. We present the results from simplified aqueous chemistry using MgCl2 brines, and compare these results with seawater. Previous studies show that different ions, e.g. Ca2+, Mg2+, \({{\rm SO}{_{4}}^{2-}}\) in the injected brine, as well as the chalk mineralogy have an impact on the stability of the rock. We performed mechanical tests on chalk cores from Liège and Stevns Klint; it was found that these two outcrop chalks exhibit an unexpected difference in their mechanical responses when comparing cores flooded with NaCl and MgCl2 at 130°C. The results of this study show that the effects of magnesium seem to be governed not only by the differences in mineralogy, but also a time dependency on chalk deformation is additionally observed. Independent of the chalk type tested, the chemical analyses performed show that when MgCl2 is flooded through the core, a significant loss of magnesium and a considerable additional amount of calcium are detected in the effluent. The experimental observations fit very well with the time-dependent chemical changes gained from the mathematical model of this study that accounts for transport effects (convection and molecular diffusion) as well as chemical processes such as precipitation/dissolution. Based on the calculations and chemical analyses, we argue that the loss of magnesium and the production of calcium cannot solely be a consequence of a substitution process. The calculations rather indicate that magnesium is precipitated forming new mineral phases and in this process not only calcite, but also silicates are dissolved. The amount of dissolved calcium and silicon from the rock matrix is significant and could thus cause an additional deformation to take place. Both the retention of magnesium in the chalk core and the formation of newly precipitated magnesium-bearing carbonates and/or magnesium-bearing clay-like minerals after flooding with MgCl2 brine were demonstrated using scanning electron microscopic methods. In addition, precipitation of anhydrite as a result of flooding with seawater-like brine was proven. The water-induced strain not only depends on the ion composition of the injected brine; moreover, the presence of non-carbonate minerals will most likely also have a significant influence on the mechanical behaviour of chalk.
Similar content being viewed by others
References
Bouillard N., Eymard R., Herbin R., Montarnal P.: Diffusion with dissolution and precipitation in a porous medium: mathematical analysis and numerical approximation of a simplified model. M2AN 41(6), 975–1000 (2007)
Davis J.A., Kent D.B.: Surface complexation modeling in aqueous geochemistry. Rev. Mineral. Geochem. 23(1), 177–260 (1990)
Evje S., Hiorth A, Madland M.V., Korsnes R.I.: A mathematical model relevant for weakening of chalk reservoirs due to chemical reactions networks and heterogeneous media. Netw. Heterog. Media 4(4), 755–788 (2009)
Heggheim T., Madland M.V., Risnes R., Austad T.: A chemical induced enhanced weakening of chalk by seawater. J. Pet. Sci. Eng. 46, 171–184 (2005)
Hiorth, A., Cathles, L.M., Kolnes, J., Vikane, O., Lohne, A., Madland, M.V.: Chemical modelling of wettability change in carbonate rocks. In: 10th Wettability Conference, Abu Dhabi, UAE, pp. 1–9 (2008a)
Hiorth, A., Cathles, L.M., Kolnes, J., Vikane, O., Lohne, A., Korsnes, R.I., Madland, M.V.: A chemical model for the seawater-CO2-carbonate system—aqueous and surface chemistry. In: International Symposium of the Society of Core Analysts, Abu Dhabi, UAE, SCA2008-18, pp. 1–12 (2008b)
Hiorth, A., Cathles, L.M., Madland, M.V.: The impact of pore water chemistry on carbonate surface charge and oil wettability. Transp. Porous Med. doi:10.1007/s11242-010-9543-6 (2010)
Hjuler M.L., Fabricius I.L.: Engineering properties of chalk related to diagenetic variations of Upper Cretaceous onshore and offshore chalk in the North Sea area. J. Pet. Sci. Eng. 68, 151–170 (2009)
Korsnes, R.I.: Chemical induced water weakening of chalk by fluid-rock interaction. A mechanistic study. PhD thesis, University of Stavanger. ISBN 978-82-7644-316-5. ISSN 1890-1387 (2007)
Korsnes R.I., Strand S., Hoff Ø., Pedersen T., Madland M.V., Austad T.: Does the chemical interaction between seawater and chalk affect the mechanical properties of chalk?. In: Cottheim, A.V., Charlier, R., Thimus, J.F., Tshibangu, J.P. (eds) Eurock 2006a, Multiphysics Coupling and Long Term Behaviour in Rock Mechanics, pp. 427–434. Taylor & Francis, London (2006a)
Korsnes R.I., Madland M.V., Austad T.: Impact of brine composition on the mechanical strength of chalk at high temperature. In: Cottheim, A.V., Charlier, R., Thimus, J.F., Tshibangu, J.P. (eds) Eurock 2006b, Multiphysics Coupling and Long Term Behaviour in Rock Mechanics, pp. 133–140. Taylor & Francis, London (2006b)
Korsnes R.I., Madland M.V., Austad T., Haver S., Røsland G.: The effects of temperature on the water weakening of chalk by seawater. J. Pet. Sci. Eng. 60(3–4), 183–193 (2008)
Madland M.V.: Water weakening of chalk. A mechanistic study. PhD thesis, University of Stavanger. ISBN 82-7644-257-9. ISSN 1502-3877 (2005)
Madland, M.V., Midtgarden, K. Manafov, R. Korsnes, R.I. Kristiansen, T.G., Hiorth, A.: The effect of temperature and brine composition on the mechanical strength of Kansas chalk. In: International Symposium of the Society of Core Analysts, Abu Dhabi, UAE, SCA2008-55, pp. 1–6 (2008)
Parkhurst, D.L., Appelo, C.A.J.: User’s guide to PHREEQC (version 2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol. Survey, Water-Resour. Invited Report, 99-4259 (1999)
Puntervold T., Strand S., Austad T.: New method to prepare outcrop chalk cores for wettability and oil recovery studies at low initial water saturation. Energy Fuels 21(6), 3425–3430 (2007)
Steefel C.I., Lasaga A.C.: A coupled model for transport of multiple chemical species and kinetic precipitation/dissolution reactions with application to reactive flow in single phase hydrothermal systems. Am. J. Sci. 294, 529–592 (1994)
Zangiabadi, B., Korsnes, R.I., Hildebrand-Habel, T., Hiorth, A., Surtarjana, I.K., Lian, A., Madland, M.V.: Chemical water weakening of various outcrop chalks at elevated temperature. In: Ling, H.I., Smyth, A., Betti, R. (eds.) Poromechanics IV: Proceedings of the 4th Biot Conference on Poromechanics, pp. 543–548. DEStech Publication, Inc., PA, USA (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madland, M.V., Hiorth, A., Omdal, E. et al. Chemical Alterations Induced by Rock–Fluid Interactions When Injecting Brines in High Porosity Chalks. Transp Porous Med 87, 679–702 (2011). https://doi.org/10.1007/s11242-010-9708-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-010-9708-3