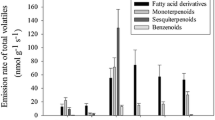
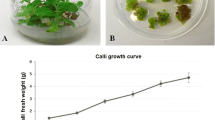
Abstract
Anemia tomentosa var. anthriscifolia is an aromatic fern with a pleasant woody aroma and antimycobacterial activity. In this paper, we describe for the first time its spore-derived gametophyte development and the effect of indole-3-acetic acid and jasmonic acid on in vitro gametophyte/sporophyte development, as well as volatile compound production. Volatiles were obtained by simultaneous distillation and extraction (SDE) and analyzed by high resolution gas chromatography coupled with mass spectrometry. Spore-derived gametophytes were able to develop into sporophytes independently of the media culture composition, even when no plant growth regulator was added. Fifty different substances were detected in all in vitro A. tomentosa SDE extracts, while 20 were detected in the wild SDE plant extract. Monoterpenes were more prevalent (69.8–89.8%) than sesquiterpenes (9.4–28.7%) in in vitro plants, while sesquiterpenes represent 97.5% of the volatiles produced by the wild-grown plants. The major monoterpene components in in vitro plants were α-pinene (9.3–24.3%), trans-pinocarveol (20.6–27.9%), pinocarvone (15.4–25.1%) and myrtenyl acetate (6.4–12.3%). The triquinane sesquiterpenes silphiperfol-6-ene (0.6–2.9%), α-guaiene (0.5–2.5%), β-barbatene (1.1–3.9%) and 9-epi-presilphiperfolan-1-ol (2.5–5.6%) represent the most abundant sesquiterpenes. The changes in the monoterpene/sesquiterpene rates between micropropagated and wild plants are not related to the presence of JA or IAA in the media culture. Further studies are still needed to obtain a complete understanding of the factors leading to these results, which could be related to differences in the irradiance levels of in vitro plants versus those from a wild environment, as well as the developmental stage of the plants. This is the first report of the use of plant growth regulators on Anemia tomentosa in vitro culture development and their effects on volatile profiles.




Similar content being viewed by others
Abbreviations
- SDE:
-
Simultaneous distillation and extraction
- HRGC-MS:
-
High resolution gas chromatography coupled with mass spectrometry
- PGR:
-
Plant growth regulator
- IAA:
-
Indole-3-acetic acid
- JA:
-
Jasmonic acid
- MS:
-
Murashige and Skoog (1962) medium
- GC:
-
Gas chromatography
- FID:
-
Flame ionization detector
- LRI:
-
Linear retention indices
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Pub. Corp, Carol Stream
Azam M, Jiang Q, Zhang B, Xu C, Chen K (2013) Citrus leaf volatiles as affected by developmental stage and genetic type. Int J Mol Sci 14:17744–17766
Bassolino L, Giacomelli E, Giovanelli S, Pistelli L, Cassetti A, Damonte G, Bisio A, Ruffoni B (2014) Tissue culture and aromatic profile in Salvia dolomitica Codd. Plant Cell Tissue Organ Cult 1–13
Bharati SK, Manabendra DT, Behari MP (2013) In vitro propagation in pteridophytes: a review. Int J Res Ayurveda Pharm 4:297–303
Camloh M, Ravnikar M, Zel J (1996) Jasmonic acid promotes division of fern protoplasts, elongation rhizoids and early development of gametophytes. Physiol Plant 97:659–664
Camloh M, Vilhar B, Zel J, Ravnlkar M (1999) Jasmonic acid stimulates development of rhizoids and shoots in fern leaf culture. J Plant Physiol 155:798–801
Chaintreau A (2001) Simultaneous distillation–extraction: from birth to maturity—review. Flavour Fragr J 16:136–148
Chang X, Alderson PG, Wright CJ (2008) Solar irradiance level alters the growth of basil (Ocimum basilicum L.) and its content of volatile oils. Environ Exp Bot 63:216–223
Das S, Choudhury MD, Mazumder PB (2013) In vitro propagation of Cyathea gigantea (Wall ex. Hook)—a tree fern. Int J Rec Scient Res 4:221–224
De Geyter N, Gholami A, Goormachtig S, Goossens A (2012) Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2012.03.001
Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J (2005) The non mevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102:933–938
Fernández H, Revilla MA (2003) In vitro culture of ornamental ferns. Plant Cell Tissue Organ Cult 73:1–13
Grausgruber-Gröger S, Schmiderer C, Steinborn R, Novak J (2012) Seasonal influence on gene expression of monoterpene synthases in Salvia officinalis (Lamiaceae). J Plant Physiol 169:353–359
Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J Biol Chem 278:26666–26676
Hemmerlin A, Harwood JL, Bach TJ (2012) A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog Lipid Res 51:95–148
Hou G, Hill JP, Blancaflor EB (2004) Developmental anatomy and auxin response of lateral root formation in Ceratopteris richardii. J Exp Bot 55:685–693
Juliani HR, Zygadlo JA, Scrivanti R, Sota E, Simon JE (2004) The essential oil of Anemia tomentosa (Savigny) Sw. var. anthriscifolia (Schard.) Mickel. Flavour Fragr J 19:541–543
Kodym A, Lang M, Delpratt J (2016) Propagation by partial tissue culture of Austral Bracken (Pteridium esculentum) for revegetation. Ecol Manag Restor 17:159–163
Kosakivska IV, Babenko LM, Shcherbatiuk MM, Vedenicheva NP, Voytenko LV, Vasyuk VA (2016) Phytohormones during growth and development of Polypodiophyta. Adv Biol Earth Sci 1:26–44
Kuriyama A, Kawai F, Kanamori M, Dathe W (1993) Inhibitory effect of jasmonic acid on gametophytic growth, initiation and development of sporophytic shoots in Equisetum arvense. J Plant Physiol 141:694–697. https://doi.org/10.1016/S0176-1617(11)81576-2
Makowski D, Tomiczak K, Rybczynski JJ, Mikuła A (2016) Integration of tissue culture and cryopreservation methods for propagation and conservation of the fern Osmunda regalis L. Acta Physiol Plant. https://doi.org/10.1007/s11738-015-2037-y
Menéndez V, Arbesú R, Somer M (2010) From spore to sporophyte: how to proceed in vitro. In: Fernandéz H, Kumar A, Revilla A (eds) Working with ferns: issues and applications, 1st edn. Springer, New York, pp 97–110
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tabaco tissue cultures. Physiol Plant 15:473–497
Nagegowda DA (2010) Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett 584:2965–2973. https://doi.org/10.1016/j.febslet.2010.05.045
NIST/EPA/NIH mass spectral library (NIST8S and NIST08.LIB) and NIST MS Search/Analysis Program and data for Microsoft Windows. (2008) Version 8 and 8 s. Shimadzu
Pangesti N, Pineda A, Pieterse CM, Dicke M, van Loon JJ (2013) Two-way plant mediated interactions between root-associated microbes and insects: from ecology to mechanisms. Front Plant Sci 23:1–11. https://doi.org/10.3389/fpls.2013.00414
Parajuli J, Joshi SD (2014) In vitro study of effects of growth hormones on sporophyte development of Cyathea spinulosa. Int J Biodivers Conserv 6:247–255. https://doi.org/10.5897/IJBC2014.0684
Parthier B (1996) Jasmonates: hormonal regulators or stress factors in leaf senescence? J Plant Growth Regul 9:57–63
Pinto SC, Leitão GG, Bizzo HR, Martinez N, Dellacassa E, Santos FM Jr, Costa FLP, de Amorim MB, Leitão SG (2009a) Epi-presilphiperfolan-1-ol, a new triquinane sesquiterpene from the essential oil of Anemia tomentosa var. anthriscifolia (Pteridophyta). Tetrahedron Lett 50:4785–4787
Pinto SC, Leitão GG, de Oliveira DR, Bizzo HR, Ramos DF, Coelho TS, Silva PE, Lourenço MC, Leitão SG (2009b) Chemical composition and antimycobacterial activity of the essential oil from Anemia tomentosa var. anthriscifolia. Nat Prod Commun 4:1675–1678
Pinto SC, Leitão GG, Castellar A, Bizzo HR, Leitão SG (2013) Chemical composition of the volatile fractions from wild and in vitro plants of Anemia tomentosa var. anthriscifolia (Pteridophyta). J Essent Oil Res 25:198–202
Praptosuwiryo TN (2017) Spore germination and early gametophyte development of Platycerium wandae (Polypodiaceae) from Papua, Indonesia. Biodiversitas 18:175–182. https://doi.org/10.13057/biodiv/d180124
Santos MG, Rocha LM, Carvalho ES, Kelecom A (2006) Isoafricanol, um sesquiterpeno incomum encontrado na Pteridófita Anemia tomentosa var. anthriscifolia. Rev Bras Plantas Med 8:71–75
Schwartsburd PB, Labiak PH (2007) Pteridófitas do Parque Estadual de Vila Velha, Ponta Grossa, Paraná, Brasil. Hoehnea 34:159–209
Sommer M, Arbesu R, Menendez V, Revilla MA, Fernandez H (2010) Sporophyte induction studies in ferns in vitro. Euphytica 171:203–210
van Schie CCN, Haring MA, Schuurink RC (2007) Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol 64:251–263. https://doi.org/10.1007/s11103-007-9149-8
Wasternack C (2014) Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol Adv 32:31–39
Wu H, Xiu-Qun L, Hua J, Long-Qing C (2010) Effects of light, macronutrients, and sucrose on germination and development of the endangered fern Adiantum reniforme var. sinense (Adiantaceae). Sci Hortic 125:417–421
Acknowledgements
Authors wish to thank Profs. Leandro Soter de Mariz e Miranda and Rodrigo Octavio Mendonça from Instituto de Química, UFRJ for the use of the GC–FID and GC–MS equipment, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for funding.
Author information
Authors and Affiliations
Contributions
JFFN, CVVC and CSB conducted the experiments; SCP and CVVC analyzed all chromatographic data, substance identification and assembled the supplementary data; SGL and NCBS conceived this work, analyzed the data and wrote the paper. All authors contributed in the writing of this paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castilho, C.V.V., Neto, J.F.F., Leitão, S.G. et al. Anemia tomentosa var. anthriscifolia in vitro culture: sporophyte development and volatile compound profile of an aromatic fern. Plant Cell Tiss Organ Cult 133, 311–323 (2018). https://doi.org/10.1007/s11240-018-1383-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1383-z