Abstract
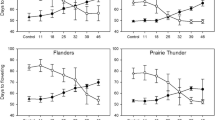
Understanding the role light quality plays on floral initiation is key to a range of pre-breeding tools, such as accelerated single-seed-descent. We have elucidated the effect of light quality on early flowering onset in cool-season grain legumes and developed predictive models for time to flowering under the optimised light conditions. Early and late flowering genotypes of pea, chickpea, faba bean, lentil and lupin were grown in controlled environments under different light spectra (blue and far red-enriched LED lights and metal halide). All species and genotypes showed a positive response to a decreasing red to far-red ratio (R:FR). In general, ratios above 3.5 resulted in the longest time to flowering. In environments with R:FR below 3.5, light with the highest intensity in the FR region was the most inductive. We demonstrate the importance of considering both relative (R:FR) and absolute (FR photons) light values for flower induction in grain legumes. Greater response to light spectra was observed in the later flowering genotypes, enabling a drastic compression of time to flowering between phenologically diverse genotypes. A novel protocol for robust in vitro germination of immature seeds was developed for lupin, a species known for its recalcitrance to in vitro manipulation. We show how combining this protocol with growth under conditions optimized for early flowering drastically speeds generation turnover. The improved understanding of the effect of light on flowering regulation and the development of robust in vitro culture protocols will assist the development and exploitation of biotechnological tools for legume breeding.


Similar content being viewed by others
References
Ausín I, Alonso-Blanco C, Martinez-Zapater JM (2005) Environmental regulation of flowering. Int J Dev Biol 49:689–705
Bayliss KL, Wroth JM, Cowling WA (2004) Pro-embryos of Lupinus spp. produced from isolated microspore culture. Aust J Agric Res 55:589–593
Bermejo C, Gatti I, Cointry E (2016) In vitro culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tissue Organ Cult. In press, doi:10.1007/s11240-016-1065-7
Clarke HJ, Wilson JG, Kuo I, Lülsdorf MM, Mallikarjuna N, Kuo J, Siddique KHM (2006) Embryo rescue and plant regeneration in vitro of selfed chickpea (Cicer arietinum L.) and its wild annual relatives. Plant Cell Tissue Organ Cult 85:197–204
Cope KR, Bugbee B (2013) Spectral effects of three types of white light-emitting diodes on plant growth and development: absolute versus relative amounts of blue light. HortScience 48:504–509
Croser JS, Lulsdorf MM, Davies PA, Clarke HJ, Bayliss KL, Mallikarjuna N, Siddique KHM (2006) Toward doubled haploid production in the Fabaceae: Progress, constraints, and opportunities. Crit Rev Plant Sci 25:139–157
Croser J, Ribalta F, Navarro MP, Munday C, Nelson K, Edwards K, Castello M, Bennett R, Erskine W (2015) Accelerated Single Seed Descent (aSSD)–a novel breeding technique to speed attainment of homozygosity. Proceedings 2nd international symposium on agricultural technology (ISAT2015), Bangkok, Thailand, 30 June
Cummings IG, Reid JB, Koutoulis A (2007) Red to far-red ratio correction in plant growth chambers—growth responses and influence of thermal load on garden pea. Physiol Plant 131:171–179
Erskine W, Hussain A, Tahir M, Bahksh A, Ellis RH, Summerfield RJ, Roberts EH (1994) Field evaluation of a model of photothermal flowering responses in a world lentil collection. Theor Appl Genet 88:423–428
Franklin G, Pius PK, Ignacimuthu S (2000) Factors affecting in vitro flowering and fruiting of green pea (Pisum sativum L.) Euphytica 115:65–74
Gallardo K, Kurt C, Thompson R, Ochatt S (2006) In vitro culture of immature M. truncatula grains under conditions permitting embryo development comparable to that observed in vivo. Plant Sci 170:1052–1058
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Lulsdorf MM, Croser JS, Ochatt S (2011) Androgenesis and doubled-haploid production in food legumes. In: Pratap A, Kumar J (eds) Biology and breeding of food legumes. CABI Publishing, Cambridge, pp 159–177
Mallikarjuna N (2003) Wide hybridization in important food legumes. In: Improvement strategies of Leguminosae biotechnology. Springer, Dordrecht, pp 155–171
Massa GD, Kim H-H, Wheeler RM, Mitchell CA (2008) Plant productivity in response to LED lighting. HortScience 43:1951–1956
Mobini SH, Warkentin TD (2016) A simple and efficient method of in vivo rapid generation technology in pea (Pisum sativum L.). In Vitro Cell Dev Biol. in press, doi:10.1007/s11627-016-9772-7
Mobini SH, Lulsdorf M, Warkentin TD, Vandenberg A (2015) Plant growth regulators improve in vitro flowering and rapid generation advancement in lentil and faba bean. In Vitro Cell Dev Biol 51:71–79
Mobini S, Lulsdorf M, Warkentin T, Vandenberg A (2016) Low red: far-red light ratio causes faster in vitro flowering in lentil. Can J Plant Sci. doi:10.1139/CJPS-2015-0282
Moe R, Heins R (1990) Control of plant morphogenesis and flowering by light quality and temperature. Acta Hortic 272:81–89
Naznin MT, Lefsrud MG (2014) Impact of LED irradiance on plant photosynthesis and action spectrum of plantlet. Proceedings SPIE optical engineering and applications. Int Soc Opt Photonics 19:921602–921605
Nelson MN, Berger JD, Erskine W (2010) Flowering time control in annual legumes: Prospects in a changing global climate. CAB Rev Perspect Agric Vet Sci Nutr Nat Res 5:49–62
Ochatt SJ (2015) Agroecological impact of an in vitro biotechnology approach of embryo development and seed filling in legumes. Agron Sustain Dev 35:535–552
Ochatt SJ, Sangwan RS (2010) In vitro flowering and seed set: acceleration of generation cycles. In: Davey MR, Anthony P (eds) Plant cell culture: essential methods. John Wiley & Sons, Ltd., Chichester, pp 97–110
Ochatt SJ, Sangwan RS, Marget P, Ndong YA, Rancillac M, Perney P (2002) New approaches towards the shortening of generation cycles for faster breeding of protein legumes. Plant Breed 121:436–440
Palmer JL, Lawn RJ, Adkins SW (2002) An embryo-rescue protocol for Vigna interspecific hybrids. Aust J Bot 50:331–338
Ribalta FM, Croser JS, Erskine W, Finnegan PM, Lulsdorf MM, Ochatt SJ (2014) Antigibberellin-induced reduction of internode length favors in vitro flowering and seed-set in different pea genotypes. Biol Plant 58:39–46
Ribalta FM, Pazos-Navarro M, Nelson K, Edwards K, Ross JJ, Bennett RG, Munday C, Erskine W, Ochatt SJ, Croser JS (2016) Precocious floral initiation and identification of exact timing of embryo physiological maturity facilitate germination of immature seeds to truncate the lifecycle of pea. Plant Growth Regul. doi:10.1007/s10725-016-0211-x
Rüdiger W, Thümmler F (2012) The phytochrome chromophore. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants. Kluwer Acad Publ, Dordretch, pp 51–67
Runkle ES, Heins RD (2001) Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J Am Soc. Hort Sci 126:275–282
Smith H (1994) Sensing the light environment: the functions of the phytochrome family. In: Photomorphogenesis in plants. Springer, Dordrecht, pp 377–416
Spalding EP, Folta KM (2005) Illumination topics in plant photobiology. Plant Cell Environ 28:39–53
Stutte GW (2009) Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 44:231–234
Summerfield RJ, Roberts EH, Erskine W, Ellis RH (1985) Effects of temperature and photoperiod on flowering in lentils (Lens culinaris Medic.) Ann Bot 56:659–671
Summerfield RJ, Roberts EH, Ellis RH, Lawn RJ (1991) Towards the reliable prediction of time to flowering in six annual crops. I. The development of simple models for fluctuating field environments. Exp Agric 27:11–31
Surma M, Adamski T, Swiecicki W, Barzyk P, Kaczmarek Z, Kuczynska A, Krystkowiak K, Mikolajczak K, Ogrodowicz P (2013) Preliminary results of in vitro culture of pea and lupin embryos for the reduction of generation cycles in single seed descent technique. Acta Soc Bot Pol 82:231–236
Weller JL, Ortega R (2015) Genetic control of flowering time in legumes. Front. Plant Sci 6:1–13
Weller JL, Beauchamp N, Kerckhoffs LHJ, Platten JD, Reid JB (2001) Interaction of phytochromes A and B in the control of de-etiolation and flowering in pea. Plant J 26:283–294
Wolko B, Clements JC, Naganowska B, Nelson MN, Yang Ha (2011) Lupinus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources: legumecrops and forages. Springer, Berlin, pp 153–206
Acknowledgments
This work was supported by the Grains Research and Development Corporation [UWA00159]. We thank Mr B. Piasini and Mr L. Hodgson for glasshouse expertise and Ms Christine Munday and Ms Simone Wells for technical assistance.
Author contributions
JSC, MPN, FMR conducted experimental design, data analysis and manuscript writing. WE, RC and RGB were involved in strategic experimental input. KE conducted in vitro experiments. ST conducted glasshouse experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11240-016-1127-x.
Rights and permissions
About this article
Cite this article
Croser, J.S., Pazos-Navarro, M., Bennett, R.G. et al. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tiss Organ Cult 127, 591–599 (2016). https://doi.org/10.1007/s11240-016-1092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1092-4