Abstract
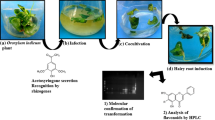
In this study, recalcitrance of tea plant ( Camellia sinensis) to Agrobacterium-mediated genetic transformation was investigated with an emphasis on specialized compounds in tea. Chemical constituents in tea leaves and calli were extracted into liquid Luria–Bertani (LB) medium to determine their biological activities on Agrobacterium growth, virulence, and plant transformation efficiency. Compared to the control Agrobacterium grown in LB medium, tea leaf extract containing 6.5 mg mL−1 catechins resulted in an 84.6 % reduction of Agrobacterium growth, a 73–36 % suppression of expression for the six virulence (vir) genes, browning of infected tobacco explant wounds, and an absence of transient or stable transformation events. Tea callus extract, containing 0.22 mg mL−1 catechins, did not significantly affect Agrobacterium growth or tobacco transgenic hairy root generation, whereas it enhanced the expression of some vir genes. Treatment with authentic catechin mixtures (other than caffeine) dissolved in LB resulted in suppression of Agrobacterium growth, vir gene expression, and tobacco transformation efficiency. Our data suggest that catechins are the key active constituents in tea leaves. Transient transformation efficiencies of tea leaves were much lower than those of tobacco leaves as indicated by the GUS (β-glucuronidase) assay, probably a result of inhibition by the catechins present in tea leaves. Lower transformation efficiencies of tea calli suggested that additional plant factor(s) might also exert inhibitory effects on tea plant transformation. Agrobacterium rhizogenes ATCC 15834 induced transgenic roots from the tea explants with 15–20 % efficiency. Our data suggested catechins inhibition of tea gene transformation could be overcome by using optimized strains of Agrobacterium.





Similar content being viewed by others
Abbreviations
- HPLC:
-
High performance liquid chromatography
- AS:
-
Acetosyringone
- GUS:
-
β-glucuronidase
- EDTA:
-
Ethylene diamine tetraacetic acid
- PCR:
-
Polymerase chain reaction
References
Ahn YJ, Kawamura T, Kim M, Yamamoto T, Mitsuoka T (1991) Tea polyphenols: selective growth inhibitors of Clostridium spp. Agric Biol Chem 55:1425–1426
Amita B, Priyanka S, Vitaly C (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719
Anand A, Krichevsky A, Schornack S, Lahaye T, Tzfira T, Tang Y, Citovsky V, Mysore KS (2007) Arabidopsis VirE2 interacting protein2 is required for Agrobacterium T-DNA integration in plants. Plant Cell 19:1695–1708
Ashby AM, Watson MD, Loake GJ, Shaw CH (1988) Tiplasmid specific chemotaxis of Agrobacterium tumefaciens C58C1 towards vir inducing phenolic compounds and soluble factors from mono cotyledons and dicotyledonous plants. J Bacteriol 170:4181–4187
Balentine DA, Wiseman SA, Bouwens LCM (1997) The chemistry of tea flavonoids. Crit Rev Food Sci 37:693–704
Collin HA, Edwards S (1998) Plant cell culture. BIOS Scientific Publisher Limited 1:10–15
Dada KD, Tripathy L (2005) Agrobacterium-induced hypersensitive necrotic reaction in plant cells: a resistance response against Agrobacterium-mediated DNA transfer. Afr J Biotechnol 4:752–757
Deng WW, Wang S, Chen Q, Zhang ZZ, Hu XY (2012) Effect of salt treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Physiol Biochem 56:35–40
Dixon RA, Pasinetti GM (2010) Flavonoids and isoflavonoids: from plant biology to agriculture and neuroscience. Plant Physiol 154:453–457
Dunnett CW (1955) A multiple comparison procedure for comparing several treatments with a control. J Am Statist Assoc 50:1096–1121
Feng L, Hou RY, Zhang L, Wan XC, Gao MJ, Wei S (2014) Determination of quality constituents in the young leaves of albino tea cultivars. Food Chem 155:98–104
Forrest GI (1969) Studies on the polyphenol metabolism of tissue cultures derived from the tea plant [Camellia sinensis (L.)]. Biochem J 113:765–772
Fronzes R, Christie PJ, Waksman G (2009) The structural biology of type IV secretion systems. Nat Rev Microbiol 7:703–714
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:151–158
Gelvin SB (2000) Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu Rev Plant Physiol Plant Mol Biol 51:223–256
Hooykaas PJJ, Klapwijk PM, Nuti MP, Schilperoort RA, Rörsch A (1977) Transfer of the Agrobacterium tumefaciens TI plasmid to avirulent Agrobacteria and to Rhizobium ex planta. J Gen Microbiol 98:477–484
Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Janssen BJ, Gardner RC (1989) Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol 14:61–72
John KMM, Joshi SD, Mandal AKA, Kumar SR, Kumar RR (2009) Agrobacterium rhizogenes-mediated hairy root production in tea leaves [Camellia sinensis (L.) O. Kuntze]. Indian J Biotechnol 8:430–434
Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP (2012) Recent advances on tea polyphenols. Front Biosci (Elite Ed) 4:111–131
Kumar N, Pandey S, Bhattacharya A, Ahuja PS (2004) Do leaf surface characteristics affect Agrobacterium infection in tea (Camellia sinensis (L.) O. Kuntze). J Bioscience 29:309–317
Li XB, Cai L, Cheng NH, Liu JW (2002) Molecular characterization of the cotton GhTUB1 gene that is preferentially expressed in fiber. Plant Physiol 130:666–674
Liu YJ, Gao LP, Xia T, Zhao L (2009) Investigation of the site-specific accumulation of catechins in the tea plant (Camellia sinensis (L.) O. Kuntze) via vanillin-HCl staining. J Agric Food Chem 57:10371–10376
Livak K, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Lopez SJ, Kumar RR, Pius PK, Muraleedharan N (2004) Agrobacterium tumefaciens-mediated genetic transformation in tea (Camellia sinensis [L.] O. Kuntze). Plant Mol Biol Rep 22:201–202
Luo YY, Liang YR (2000) Studies on the construction of Bt gene expression vector and its transformation in tea plant. J Tea Sci 20:141–147
Matsumoto S, Fukui M (1998) Agrobacterium tumefaciens medaited gene transfer in tea plant (Camellia sinensis) cells. Jpn Agric Res Q 32:287–291
Medina-Bolivar F, Condori J, Rimando AM, Hubstenberger J, Shelton K, O’Keefe SF, Bennett S, Dolan MC (2007) Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 68:1992–2003
Mohanpuria P, Kumar V, Ahuja PS, Yadav SK (2011) Agrobacterium-mediated silencing of caffeine synthesis through root transformation in Camellia sinensis L. Mol Biotechnol 48:235–243
Mohanpuria P, Rana NK, Yadav SK (2008) Transient RNAi based gene silencing of glutathione synthetase reduces glutathione content in Camellia sinensis (L.) O. Kuntze somatic embryos. Biol Plantarum 52(2):381–384
Mondal TK, Bhattacharya A, Ahuja PS, Chand PK (2001a) Transgenic tea [Camellia sinensis (L.) O. Kuntze cv. Kangra Jat] plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep 20:712–720
Mondal TK, Bhattacharya A, Ahuja PS, Chand PK (2001b) Factor effecting Agrobacterium tumefaciens mediated transformation of tea (Camellia sinensis (L). O.Kuntze). Plant Cell Rep 20:712–720
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Pang Y, Abeysinghe ISB, He J, He X, Huhman D, Mewan KM, Sumner LW, Yun J, Dixon RA (2013) Functional characterization of proanthocyanidin pathway enzymes from tea and their application for metabolic engineering. Plant Physiol 161:1103–1116
Pitzschke A (2013) Agrobacterium infection and plant defense-transformation success hangs by a thread. Front Plant Sci 4:519
Sandal I, Kumar A, Bhattacharya A, Desikalhar RS, Gulati A, Ahuja PS (2001) A thermolabile caffeine fraction of tea leaves–A substitute of acetosyringone for Agrobacterium-mediated genetic transformations (Patent filed in US and PCT)
Sandal I, Saini U, Lacroix B, Bhattacharya A, Ahuja PS (2007) Agrobacterium-mediated genetic transformation of tea leaf explants: effects of counteracting bactericidity of leaf polyphenols without loss of bacterial virulence. Plant Cell Rep 26:169–176
Schrammeijer B, Beijersbergen A, Idler KB, Melchers LS, Thompson DV, Hooykaas PJ (2000) Sequence analysis of the vir-region from Agrobacterium tumefaciens octopine Ti plasmid pTi15955. J Exp Bot 51:1167–1169
Shibasaki-Kitakawa N, Takeishi J, Yonemoto T (2003) Improvement of catechin productivity in suspension cultures of tea callus cells. Biotechnol Prog 19:655–658
Wu XX, Li J, Wang ZK, Liu SS, Li HH, Ma Y, Li WB (2010) Effect of AS concentration and pH on soybean genetic transformation. J Northeast Agric Univ 41:1–4
Yam TS, Shah S, Hamilton-Miller JM (1997) Microbiological activity of whole and fractionated crude extract of tea (Camellia sinensis), and of tea components. FEMS Microbiol Lett 152:169–174
Yuan ZC, Edlind MP, Liu P, Saenkham P, Banta LM, Wise AA, Ronzone E, Binns AN, Kerr K, Nester EW (2007) The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci USA 104:11790–11795
Zupan JR, Zambryski P (1995) Transfer of T-DNA from Agrobacterium to the plant cell. Plant Physiol 107:1041–1047
Acknowledgments
We thank Prof Li-Ping Gao at the Anhui Agricultural University and Prof. Jianliang Lu at the Zhejiang University for providing tea calli and the Agrobacterium strains. This work was financially supported by the National Science Foundation in China (#31070614 and #31370687), the Doctoral Programs of Higher Education of the Ministry of Education (#20123418110002), the Program for Changjiang Scholars and Innovative Research Team in Universities (IRT1101), and the “Twelfth Five-Year” National Key Basic Research and Development Project (973) in China (2012CB722903).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, DP., Feng, L., Rana, M.M. et al. Effects of catechins on Agrobacterium-mediated genetic transformation of Camellia sinensis . Plant Cell Tiss Organ Cult 119, 27–37 (2014). https://doi.org/10.1007/s11240-014-0511-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0511-7