Abstract
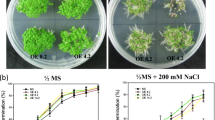
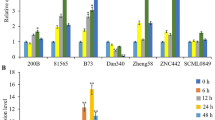
The TIR1/AFB F-box proteins act as auxin receptors and play an important role in auxin mediated plant development. In the present study, a homologue of AFB2 gene, ZmAFB2, has been cloned from maize (Zea mays L.). Expression of ZmAFB2 is induced by NaCl and salicylic acid treatment and reduced by indole-3-acetic acid (IAA) treatment. To identify the function of the ZmAFB2 gene under stress conditions, a binary vector containing ZmAFB2 driven by CaMV 35S promoter was constructed and transformed into tobacco (Nicotiana tabacum) by Agrobacterium-mediated transformation. The transgenic tobacco lines overexpressing ZmAFB2 exhibit increased tolerance to salt treatment. Furthermore, overexpression of ZmAFB2 enhances the transcription levels of stress responsive genes Ascorbate peroxidase and Catalase. These results indicate that ZmAFB2 is involved in abiotic stress response and overexpression of ZmAFB2 enhances salt tolerance in transgenic tobacco.







Similar content being viewed by others
References
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43:545–580
Calderon-Villalobos LI, Tan X, Zheng N, Estelle M (2010) Auxin perception-structural insights. Cold Spring Harb Perspect Biol 2:a005546
Cecchetti V, Altamura MM, Falasca G, Costantino P, Cardarelli M (2008) Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 20(7):1760–1774
Chen H, Li Z, Xiong L (2012) A plant microRNA regulates the adaptation of roots to drought stress. FEBS Lett 586:1742–1747
Dharmasiri N, Estelle M (2004) Auxin signaling and regulated protein degradation. Trends Plant Sci 9:302–308
Dharmasiri N, Dharmasiri S et al (2005a) Plant development is regulated by a family of auxin receptor F-box proteins. Dev Cell 9(1):109–119
Dharmasiri N, Dharmasiri S, Estelle M (2005b) The F-box protein TIR1 is an auxin receptor. Nature 435:441–445
Dowd C, Wilson IW, McFadden H (2004) Gene expression profile changes in cotton root and hypocotyl tissues in response to infection with Fusarium oxysporum f. sp. Vasinfectum. Mol Plant Microbe Interact 17(6):654–667
Faize M, Faize L, Burgos L (2010) Using quantitative real-time PCR to detect chimeras in transgenic tobacco and apricot and to monitor their dissociation. BMC Biotechnol 10:53. doi:10.1186/1472-6750-10-53
Feng XM, You CX, Qiao Yu, Mao K, Hao YJ (2010) Ectopic overexpression of Arabidopsis AtmiR393a gene changes auxin sensitivity and enhances salt resistance in tobacco. Acta Physiol Plant 32:997–1003
Friml J (2003) Auxin transport - shaping the plant. Curr Opin Plant Biol 6(1):7–12
Gao P, Bai X, Yang L, Lv DK, Pan X, Li Y, Cai H, Ji W, Chen Q, Zhu YM (2011) osa-MIR393: a salinity- and alkaline stress-related microRNA gene. Mol Biol Rep 38:237–242
Geraint P, Mark E (2006) Auxin receptors: a new role for F-box proteins. Curr Opin Cell Biol 18:152–156
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Goodman CD, Casati P, Walbot V (2004) A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell 16:1812–1826
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414:271–276
Haglund K, Dikic I (2005) Ubiquitylation and cell signaling. EMBO J 24:3353–3359
He Y, Liu Y, Cao W, Huai M, Xu B, Huang B (2005) Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentuky Bluegrass. Crop Sci 45:988–995
Huang WL, Lee CH, Chen YR (2012) Levels of endogenous abscisic acid and indole-3-acetic acid influence shoot organogenesis in callus cultures of rice subjected to osmotic stress. Plant Cell Tiss Organ Cult 108:257–263
Iglesias MJ, Terrile MC, Bartoli CG, D’Ippólito S, Casalongué CA (2010) Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism in Arabidopsis. Plant Mol Biol 74(3):215–222
Iglesias MJ, Terrile MC, Casalongué CA (2011) Auxin and salicylic acid signalings counteract the regulation of adaptive responses to stress. Plant Signal Behav 6(3):452–454
Jain M, Khurana JP (2009) Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J 276:3148–3162
Jain S, Kumar D, Jain M, Chaudhary P, Deswal R, Sarin NB (2012) Ectopic overexpression of a salt stress-induced pathogenesis-related class 10protein (PR10) gene from peanut (Arachis hypogaea L.) affords broad spectrum abiotic stress tolerance in transgenic tobacco. Plant Cell Tiss Organ Cult 109:19–31
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress induced miRNA. Mol Cell 14(6):787–799
Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435:446–451
Kim J, Harter K, Theologis A (1997) Protein–protein interactions among the Aux/IAA proteins. PNAS 22:11786–11791
Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146:839–844
Letunic I, Doerks T, Bork P (2009) SMART 6: recent updates and new developments. Nucl Acids Res 37:229–232
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
O’Donnell PJ, Schmelz EA, Moussatche P (2003) Susceptible to intolerance—a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J 33(2):245–257
Park JE, Park JY, Kim YS (2007) GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem 282:10036–10046
Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M (2009) Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci 706:22540–22545
Raskin I (1992) Role of salicylic-acid in plants. Annu Rev Plant Biol 43:439–463
Ren ZX, Li ZG, Miao Q, Yang YW, Deng W, Hao YW (2011) The auxin receptor homologue in Solanum lycopersicum stimulates tomato fruit set and leaf morphogenesis. J Exp Bot 62(8):2815–2826
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108
Shen CJ, Bai YH, Wang SK, Zhang SN, Wu YR, Chen M, Jiang DA, Qi YH (2010) Expression profile of PIN, AUX/LAX and PGP auxin transporter gene families in Sorghum bicolor under phytohormone and abiotic stress. FEBS J 277:2954–2969
Singh B, Usha K (2003) Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul 39:137–141
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N (2007) Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446:640–645
Tasgin E, Atici O, Nalbantoghu B (2003) Effect of salicylic acid and cold on freezing tolerance in wheat leaves. Plant Growth Regul 41:231–236
Tian Y, Zhang CH, Yang H, Lu XY, Fang J, Li PW, Qing XG (2011) Molecular cloning and characterization of a TRANSPORT INHIBITOR RESPONSE 1 (TIR1) from Nicotiana tabacum. Russ J Plant Physiol 58(1):149–156
Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ (2001) Aux/IAA proteins are active repressors and their stability and activity are modulated by auxin. Plant Cell 13:2809–2822
Tiwari SB, Hagen G, Guilfoyle TJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16:533–543
Topping JF (1998) Tobacco transformation. Methods Mol Biol 81:365–372. doi:10.1385/0-89603-385-6:365
Ulmasov T, Hagen G, Guilfoyle TJ (1999) Activation and repression of transcription by auxin-response factors. PNAS 96(10):5844–5849
Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2:123
Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17:1784–1790
Wang SK, Bai YH, Shen CJ, Wu YR, Zhang SN, Jiang DA, Guilfoyle TJ, Chen M, Qi YH (2010) Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct Integr Genomic 10:533–546
Xuan N, Jin Y, Zhang H, Xie Y, Liu Y, Wang G (2011) A putative maize zinc-finger protein gene, ZmAN13, participates in abiotic stress response. Plant Cell Tiss Organ Cult 107:101–112
Yusuf M, Hasan SA, Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) Effect of salicylic acid on salinity-induced changes in Brassica juncea. J Integr Plant Biol 50(9):1096–1102
Zhang ZQ, Li Q, Li ZM, Staswick PE, Wang MY, Zhu Y, He ZH (2007) Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol 145:450–464
Zhao B, Liang R, Ge L, Li W, Xiao H, Lin H, Ruan K, Jin Y (2007) Identification of drought-induced microRNAs in rice. Biochem Biophys Res Co 354(2):585–590
Zhou J, Li FX, Chen S (2010) Cloning and expression analysis of the PVY resistance-related gene NtPsaN in Tobacoo (Nicotiana tabacum). Sci Agric Sin 43(16):3323–3330
Acknowledgments
This work was supported by the Projects of National Natural Science Foundation of China (30972002, 31071798) and the Committee of Science and Technology of Chongqing (2011BA1024).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chunwen Yang and Wei Deng contributed equally to this work and are considered co-first authors.
Rights and permissions
About this article
Cite this article
Yang, C., Deng, W., Tang, N. et al. Overexpression of ZmAFB2, the maize homologue of AFB2 gene, enhances salt tolerance in transgenic tobacco. Plant Cell Tiss Organ Cult 112, 171–179 (2013). https://doi.org/10.1007/s11240-012-0219-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0219-5