Abstract
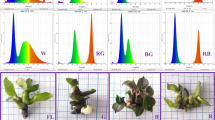
The effects of different spectral light distribution on in vitro induction and proliferation of Oncidium protocorm-like bodies (PLBs) and subsequent growth of plantlets were investigated. Shoot tips (5 mm in length) of proliferating shoots of Oncidium “Gower Ramsey” were vertically incubated on 1/2 Murashige and Skoog (MS) medium supplemented with 1.0 mg l−1 6-benzyladenine (BA), and grown under either monochromatic red light-emitting diodes (LEDs) (RR), blue LEDs (BB), yellow LEDs (YY) or green LEDs (GG). Cultures grown under fluorescent lamps (FL) were used as control. Selected FL-induced PLBs were cut into 3- to 4-mm sections and incubated on MS medium supplemented with 1.0 mg l−1 BA and 0.5 mg l−1 α-naphthaleneacetic acid (NAA), and grown under RR, BB, YY, GG, or FL. Moreover, FL-differented shoots (15 mm in length with two leaves) were incubated on 1/2 MS medium with 0.5 mg l−1 NAA, and grown under either FL, RR, 10% blue + 90% red LEDs (1BR), 20% blue + 80% red LEDs (2BR), 30% blue + 70% red LEDs (3BR), BB, 80% red + 10% blue + 10% far-red LEDs (RBFr), or 80% red + 10% blue + 10% green LEDs (RBG). Overall, the red light spectrum enhanced induction, proliferation, and the carbohydrate contents of PLBs, as well as subsequent plantlet lengths, while the blue spectrum promoted differentiation, protein accumulation, and enzyme activities in PLBs, as well as pigment content accumulation in PLBs and developing plantlets. The combination of red and blue LEDs resulted in higher energy efficiency as well as dry weight and enzyme activities in these plantlets.







Similar content being viewed by others
References
Andrea K, Francisco JZA (1999) Natural light as an alternative light source for the in vitro culture of banana. Plant Cell Tissue Organ Cult 55:141–145
Anna B, Alicja K (2001) Effect of light quality on somatic embryogenesis in Hyacinthus orientalis L. ‘Delft’s Blue’. Biol Bull Poznan 38:103–107
Arditti J, Ernst R (1993) Micropropagation of orchids. Wiley, New York
Arnon DL (1949) Copper enzymer in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Mol Biol 50:601–639
Bradford E (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Charles EH, Frederick GK (1948) The accuracy of estimation of hydrogen peroxide by potassium permanganate titration. J Am Chem Soc 70(4):1640–1644
Chen JT, Chang WC (2000a) Plant regeneration via embryo and shoot bud formation from flower-stalk explants of Oncidium ‘Sweet Sugar’. Plant Cell Tissue Organ Cult 62:95–100
Chen JT, Chang WC (2000b) Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (orchidaceae). Plant Sci 160:87–93
Chen JT, Chang WC (2002) Effects of tissue culture conditions and explant characteristics on direct somatic embryogenesis in Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult 69:41–44
Chen JT, Chang WC (2003) Effects of GA3, ancymidol, cycocel and paclobutrazol on direct somatic embryogenesis of Oncidium in vitro. Plant Cell Tissue Organ Cult 72:105–108
Cybularz-Urban T, Hanus-Fajerska E, Swiderski A (2007) Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol Crac Ser Bot 49(1):113–118
Dewir YH, Chakrabarty D, Ali MB, Hahn EJ, Paek KY (2006) Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ Exp Bot 58:93–99
Fairbairn NJ (1953) A modified anthrone reagent. Chem Ind (4):86
Hamada K, Shimassaki K, Nishimura Y, Egawa H, Yoshida K (2009) Effect of red fluorescent films on the proliferation of Cymbidium finlaysonianum Lindl. PLB cultured in vitro. Hort Environ Biotechnol 50:319–323
Huan LVT, Tanaka M (2004) Effects of red and blue light-emitting diodes on callus induction, callus proliferation, and protocorm-like body formation from callus in Cymbidium orchid. Environ Control Biol 42:57–64
Jao RC, Lai CC, Fang W, Chang SF (2005) Effects of red light on the growth of Zantedeschia plantlets in vitro and tuber formation using light-emitting diodes. HortScience 40(2):436–438
Jheng FY, Do YY, Liauh YW, Chung JP, Huang PL (2006) Enhancement of growth and regeneration efficiency from embryogenic callus cultures of Oncidium ‘Gower Ramsey’ by adjusting carbohydrate sources. Plant Sci 170:1133–1140
Kim HH, Goins GD, Wheeler RM (2004a) Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 39:1617–1622
Kim SJ, Hahn EJ, Heo JW (2004b) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of Chrysanthemum plantlets in vitro. Sci Hortic 101:143–151
Kowallik W (1982) Blue light effects on respiration. Plant Physiol 33:51–72
Kozai T, Kubota C, Jeong BR (1997) Environmental control for the large-scale production of plants through in vitro techniques. Plant Cell Tissue Organ Cult 51:49–56
Li SH, Kuoh CS, Chen YH, Chen HH, Chen WH (2005) Osmotic sucrose enhancement of single-cell embryogenesis and transformation efficiency in Oncidium. Plant Cell Tissue Organ Cult 81:183–192
Li HM, Xu ZG, Tang CM (2010) Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-010-9763-z
Lian ML, Murthy HN, Paek KY (2002) Effects of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ‘Pesaro’. Sci Hortic 94:365–370
Liang JX, Chen B (2006) Influence of light quality on seedling differentiation from Sugarcane callus. Sugar crops of China 3:9–11 (in Chinese)
Maevskaya SN, Bukhov NG (2005) Effect of light quality on nitrogen metabolism of radish plants. Russ J Plant Physiol 52(3):304–310
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nguyen QT, Kozai T, Niu G, Nguyen UV (1999) Photosynthetic characteristics of coffee (Coffeaarabusta) plantlets in vitro in response to different CO2 concentrations and light intensities. Plant Cell Tissue Organ Cult 55:133–139
Nhut DT, Takamura T, Watanabe H (2003) Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult 73:43–52
Onofrio CD, Morini S, Bellocchil G (1998) Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tissue Organ Cult 53:91–98
Pütter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, NY, pp 685–690
Schuerger AC, Brown CS, Stryjewski EC (1997) Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann Bot 79:273–282
Senge M, Senger H (1991) Adaptation of photosynthetic apparatus of Chlorella and Ankistrodesmus to blue and red light. Bot Acta 104:139–143
Sivakumar G, Heo JW, Kozai T, Peak KY (2006) Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J Hortic Sci Biotechnol 81:546–548
Su YJ, Chen JT, Chang WC (2006) Efficient and repetitive production of leaf-derived somatic embryos of Oncidium. Biologia Plantarum 50:107–110
Takahashi K, Fujino K, Kikuta Y, Koda Y (1995) Involvement of the accumulation of sucrose and the synthesis of cell wall polysaccharides in the expansion of potato cells in response to jasmonic acid. Plant Sci 111:11–18
Tanaka M, Takamura T, Watanabe H, Endo M, Yanagi T, Okamoto K (1998) In vitro growth of Cymbidium plantlets cultured under superbright red and blue light-emitting diodes (LEDs). J Hortic Sci Biotechnol 73:39–44
Tanaka M, Watanabe T, Giang DT, Tanaka M, Takamura T, Watanabe H (2001) Morphogenesis in the PLB segments of Phalaenopsis cultured under LED irradiation system (Abstract). J Jpn Soc Hortic Sci 70(Suppl. 1):306 (in Japanese)
Van Slyke DD, Dillon RT, MacFadyen DA, Hamilton P (1941) Gasometric determination of carboxyl groups in free amino acids. J Biol Chem 141:627–669
Wu IF, Chen JT, Chang WC (2004) Effects of auxins and cytokinins on embryo formation from root-derived callus of Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult 77:107–109
Xiao P, Zhong WJ, Zhang RQ (1999) The comparison of chemical luminescence and chemical colorimetric determination method of SOD. Shanghai J Prev Med 11(2):54–56 (in Chinese)
Zhang Z, Li S, Li W (2008) Effects of different light qualities on the multiplication of the callus from Vitis vinifera L. and resveratrol content. Plant Physiol Commun 44:106–108 (in Chinese)
Zhang YS, Huang X, Chen YF (2009) Experimental course of plant physiology. Higher Education Press, Beijing (in Chinese)
Zhao SJ, Xu CC, Zhou Q (1994) The plant tissue malondialdehyde determination method of improvement. Plant Physiol Commun 30(3):207–210 (in Chinese)
Acknowledgments
This project was partially funded by National Science and Technology Support Project of China (No: 2010AA03A165) and Natural Science Fundation of China (No: 30972035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mengxi, L., Zhigang, X., Yang, Y. et al. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tiss Organ Cult 106, 1–10 (2011). https://doi.org/10.1007/s11240-010-9887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9887-1