Abstract
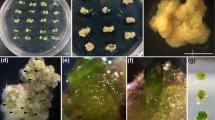
Attempts were made to study the effect of thidiazuron (TDZ) on adventitious shoot induction and plant development in Paulownia tomentosa explants derived from mature trees. Media with different concentrations of TDZ in combination with an auxin were used to induce adventitious shoot-buds in two explant types: basal leaf halves with the petiole attached (leaf explant) and intact petioles. Optimal shoot regeneration was obtained in leaf explants cultured on induction medium containing TDZ (22.7 or 27.3 μM) in combination with 2.9 μM indole-3-acetic acid (IAA) for 2 weeks, and subsequent culture in TDZ-free shoot development medium including 0.44 μM BA for a further 4-week period. The addition of IAA to the TDZ induction medium enhanced the shoot-forming capacity of explants. The caulogenic response varied significantly with the position of the explant along the shoot axis. The highest regeneration potential (85–87%) and shoot number (up to 17.6 shoots/explant) were obtained in leaf explants harvested from the most apical node exhibiting unfolded leaves (node 1). An analogous trend was also observed in intact petiole explants, although shoot regeneration ability was considerably lower, with values ranging from 15% for petioles isolated from node 1 to 5% for those of nodes 2 and 3. Shoot formation capacity was influenced by the genotype, with regeneration frequencies ranging from 50% to 70%. It was possible to root elongated shoots (20 mm) in basal medium without growth regulators; however, rooting frequency was significantly increased up to 90% by a 7-day treatment with 0.5 μM indole-3-butyric acid, regardless of the previous culture period in shoot development medium (4 or 8 weeks). Shoot quality of rooted plantlets was improved not only by IBA treatment but also by using material derived from the 4-week culture period. Regenerated plantlets were successfully acclimatized in the greenhouse 8 weeks after transplanting.


Similar content being viewed by others
Abbreviations
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthaleneacetic acid
- PGRs:
-
Plant growth regulators
- TDZ:
-
Thidiazuron
References
Bergmann BA (1998) Propagation method influences first year field survival and growth of Paulownia. New For 16:251–264. doi:10.1023/A:1006529622871
Bergmann BA, Moom H-K (1997) In vitro adventitious shoot production in Paulownia. Plant Cell Rep 16:315–319
Bergmann BA, Whetten R (1998) In vitro rooting and early greenhouse growth of micropropagated Paulownia elongata shoots. New For 15:127–138
Bonga JM, von Aderkas P (1992) In vitro culture of trees. Kluwer Academic Publishers, Dordrecht, pp 236
Brown CW, Thorpe TA (1986) Plant regeneration by organogenesis. In: Vasil IK (ed) Cell culture and somatic cell genetics of plants, vol 3. Academic Press Inc, Orlando, pp 49–65
Bunn E, Senaratna T, Sivasithamparam K, Dixon KW (2005) In vitro propagation of Eucalyptus phylacis L. Johnson and K. Hill., a critically endangered relict from western Australia. In Vitro Cell Dev Biol Plant 41:812–815. doi:10.1079/IVP2005700
Burger DW (1989) Empress tree (Paulownia tomentosa Steud). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 5, trees II. Springer, Berlin, pp 359–369
Burger DW, Liu L, Wu L (1985) Rapid micropropagation of Paulownia tomentosa. HortScience 20:760–761
Cuenca B, Ballester A, Vieitez AM (2000) In vitro adventitious bud regeneration from internode segments of beech. Plant Cell Tissue Organ Cult 60:213–220. doi:10.1023/A:1006428717309
Debnath SC (2005) A two-step procedure for adventitious shoot regeneration from in vitro-derived lingonberry leaves: shoot induction with TDZ and shoot elongation using zeatin. HortScience 40:189–192
Ding JQ, Reardon R, Wu Y, Zheng H, Fu WD (2006) Biological control of invasive plants through collaboration between China and the United States of America: a perspective. Biol Invasions 8:1439–1450. doi:10.1007/s10530-005-5833-2
Essl F (2007) From ornamental to detrimental? The incipient invasion of Central Europe by Paulownia tomentosa. Preslia 79:377–389
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119. doi:10.1007/BF01983223
Ipekci Z, Gozukirmizi N (2003) Direct somatic embryogenesis and synthetic seed production from Paulownia elongata. Plant Cell Rep 22:16–24. doi:10.1007/s00299-003-0650-5
Jones MPA, Yi Z, Murch SJ, Saxena PK (2007) Thidiazuron-induced regeneration of Echinacea purpurea L.: micropropagation in solid and liquid culture systems. Plant Cell Rep 26:13–19. doi:10.1007/s00299-006-0209-3
Kumar PP, Rao CD, Goh C-J (1998) Influence of petiole and lamina on adventitious shoot initiation from leaf explants of Paulownia fortunei. Plant Cell Rep 17:886–890. doi:10.1007/s002990050503
Lu CY (1993) The use of TDZ in tissue culture. In Vitro Cell Dev Biol Plant 29:92–96. doi:10.1007/BF02632259
Lyyra S, Lima A, Merkle S (2006) In vitro regeneration of Salix nigra from adventitious shoots. Tree Physiol 26:969–975
Marcotrigiano M, Stimart DP (1983) In vitro organogenesis and shoot proliferation of Paulownia tomentosa Steud. (Empress tree). Plant Sci Lett 31:303–310. doi:10.1016/0304-4211(83)90069-X
Mencucini M, Rugini E (1993) In vitro shoot regeneration from olive cultivar tissues. Plant Cell Tissue Organ Cult 32:283–288. doi:10.1007/BF00042290
Mullins KV, Llewellyn DJ, Hartney VJ, Strauss S, Dennis ES (1997) Regeneration and transformation of Eucalyptus camaldulensis. Plant Cell Rep 16:787–791. doi:10.1007/s002990050321
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro morphogenesis. In Vitro Cell Dev Biol Plant 34:267–275. doi:10.1007/BF02822732
Rao CD, Goh C-J, Kumar PP (1996) High frequency adventitious shoot regeneration from excised leaves of Paulownia spp. cultured in vitro. Plant Cell Rep 16:204–209. doi:10.1007/BF01890868
Song SL, Sato T, Saito A, Kihachiro O (1989) Meristematic culture of seven Paulownia species. J Jpn For Soc 71:456–459
Song SL, Suda K, Ishii K, Saito A, Ohba K (1991) Plantlet regeneration from leaf and petiole explants of in vitro-cultured Paulownia catalpifolia. J Jpn For Soc 73:60–63
Vieitez AM, San-José MC (1996) Adventitious shoot regeneration from Fagus sylvatica leaf explants in vitro. In Vitro Cell Dev Biol Plant 32:140–147. doi:10.1007/BF02822757
Yang J-C, Ho C-K, Chen Z-Z, Chang S-H (1996) Paulownia x taiwaniana (Taiwan Paulownia). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 35, trees IV. Springer, Berlin, pp 269–290
Yaycili O, Alikamanoglu S (2005) The effect of magnetic field on Paulownia tissue cultures. Plant Cell Tissue Organ Cult 83:109–114. doi:10.1007/s11240-005-4852-0
Acknowledgements
Thanks are given to M. J. Cernadas for her technical support. The study was partially funded by the Ministerio de Educación y Ciencia (Spain) through the project AGL2005-00709.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corredoira, E., Ballester, A. & Vieitez, A.M. Thidiazuron-induced high-frequency plant regeneration from leaf explants of Paulownia tomentosa mature trees. Plant Cell Tiss Organ Cult 95, 197–208 (2008). https://doi.org/10.1007/s11240-008-9433-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9433-6