Abstract
Heparin-induced thrombocytopenia (HIT) occurs in approximately 3% of patients receiving heparinoids. About 30–75% of patients with type 2 of HIT develop thrombosis as a result of platelet activation. The most important clinical symptom is thrombocytopenia. Patients with severe COVID-19 are among those receiving heparinoids. This meta-analysis performed to picture the current knowledge and results of published studies in this field. Three search engines were searched and 575 papers were found. After evaluation, 37 articles were finally selected of which 13 studies were quantitatively analyzed. The pooled frequency rate of suspected cases with HIT in 13 studies with 11,241 patients was 1.7%. The frequency of HIT was 8.2% in the extracorporeal membrane oxygenation subgroup with 268 patients and 0.8% in the hospitalization subgroup with 10,887 patients. The coincidence of these two conditions may increase the risk of thrombosis. Of the 37 patients with COVID-19 and confirmed HIT, 30 patients (81%) were treated in the intensive care unit or had severe COVID-19. The most commonly used anticoagulants were UFH in 22 cases (59.4%). The median platelet count before treatment was 237 (176–290) x 103/µl and the median nadir platelet count was 52 (31–90.5) x 103/µl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heparin-induced thrombocytopenia (HIT) is a virtually proven side effect of fractionated and unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) use when thrombosis can lead to severe morbidity and mortality [1]. It is a major health challenge in intensive care units, cardiac surgery units, and cardiac catheterization laboratories because there are numerous causes of thrombocytopenia [2]. HIT occurs as a non-immunologic type (type 1) or as drug-induced and immune-mediated thrombocytopenia (type 2). Type 2 usually occurs 4–10 days after heparinoid use. HIT is rarely associated with bleeding manifestations, despite other drug induced thrombocytopenias. Nearly 30–75% of patients with undiagnosed and untreated HIT develop thrombosis. It occurs in about 3% of patients receiving unfractionated heparin (UFH) for about two weeks [3]. HIT mortality rate is about 20%, and amputation may occur in 10% of affected patients with HIT [4]. HIT usually involves IgG antibodies, rarely IgM or IgA antibodies. These antibodies recognize complexes of heparin and platelet factor 4 [5]. After the antibodies adhere to the PF4/heparin complex, platelet activation occurs, resulting in a procoagulant reaction (release of microparticles) [6, 7]. In general, HIT is classified to two types [8, 9]. Clinical symptoms such petechial, purpura, rash, skin necrosis are nonspecific, so heparin should not be replaced unnecessarily because it has a favorable risk-benefit ratio [10].
Since the first outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019, multiple waves of COVID-19 have spread to all corners of the world. COVID-19 has caused approximately 6,681,433 deaths worldwide as of 6 January 2023 [11]. SARS-C0V-2 is considered one of the most dangerous viruses in the coronavirus family [12]. The development of thrombosis, especially venous thromboembolism in COVID19, which can be a trigger for failure of many organs, is a major cause of mortality and morbidity in COVID-19 [13, 14]. Thrombosis in COVID-19 is a multifactorial process, and SARS-Co-V-2 can cause fluctuation of many prothrombotic and coagulation factors [15,16,17,18]. Heparin and heparin-like molecules as anticoagulant and anti-inflammatory drugs with direct antiviral properties have multiple effects in COVID-19 [19, 20]. One study has shown that non-critically ill patients with COVID-19 who received heparin had longer survival [21]. On the other hand, patients treated in the intensive care unit (ICU) have been shown to develop venous thromboembolism (VTE), pulmonary embolism (PE), or deep vein thrombosis (DVT) [22]. In patients requiring ICU hospitalization, VTE or PE may be present on ICU admission or may develop during the ICU stay [23, 24]. Therefore, patients in the ICU receive a prophylactic dose of an anticoagulant, including LMWH [25]. In patients with COVID-19 admitted to the ICU, the therapeutic dose of heparin was more beneficial than the prophylactic dose of heparin in moderately ill patients [26].
It seems that patients with severe COVID-19 treated in the intensive care unit have two potential factors for thrombophilia: COVID-19 and platelet activation by heparin. Therefore, the present systematic review and meta-analysis attempted to investigate the frequency of HIT in COVID-19 and related aspects.
Materials and methods
Data source and search strategy
The authors did a systematic search in three electronic medical databases (PubMed, Scopus, and Web of Science) separately to collect relevant papers examining HIT in COVID-19 patients. The following keywords were used as part of the search strategy in each database: “heparin-induced thrombocytopenia” OR “heparin-dependent IgG antibodies” OR “platelet factor 4 heparin-induced thrombocytopenia” OR “immune heparin-induced thrombocytopenia” OR “antibody-PF4-heparin complex” OR “Antiplatelet factor 4” OR “Heparin-dependent IgG (HIT-IgG) antibodies” OR “IgG against platelet factor 4” OR “IgM against platelet factor 4” AND “COVID-19” OR “SARS-CoV-2” OR “2019-nCoV”. Two independent authors (M. R. and H. M.) searched July 26, 2022, reviewed the full text to select eligible studies, entered all articles into EndNote X7 reference manager software for screening, and deleted duplicate articles. All discrepancies in each section (screening, study selection and data extraction) were resolved in a joint meeting after each section of the research.
Selection of studies and eligibility criteria
After removing duplicate articles, articles were screened using inclusion and exclusion criteria by each author separately. Any discrepancy resolved in a joint meeting. Inclusion criteria included reports of cases or frequencies of HIT cases in patients with COVID-19 who used heparin during treatment. HIT would be verified by one of the following laboratory tests: Chemiluminescent Immunoassay (CIA), Enzyme-linked Immunosorbent Assay (ELISA), Latex Immune Turbidimetric Assay (LITA), and Particle Gel Immunoassay (PaGIA). Confirmatory tests for confirmation HIT are Serotonin Release Assay (SRA) and Heparin Induced Platelet Activation (HIPA).
Exclusion criteria include review articles, case reports, commentaries, guidelines, non-English language texts, and non-use of heparin during treatment, HIT post COVID-19 vaccine injection, and studies that did not have sufficient data. Case reports merely collected to have an imagination and estimation of frequency of HIT in COVID-19. They were not entered into the equation of meta-analysis to prevent bias. The severe COVID-19 patients are those require hospitalization, whereas the non-severe COVID-19 patients are those don’t need hospitalized. Given that all patients with HIT are those had admitted in hospital, they would all be with severe COVID-19. Hence, we could not collect data on severity of patients with mild and moderate COVID-19.
Data extraction and quality assessment
To avoid bias, two reviewers (M. R. and H. M.) independently extracted data from the finally included studies: first author’s name, year of publication, country, number of patients, age and sex distribution of patients, severity of COVID-19, clinical and laboratory characteristics and outcomes of patients, frequency of HIT, if reported. To assess the quality of the studies, each study was scored 0–10. The risk of bias score was determined by summing the scores. The final score of 0–3 was low, 4–6 was moderate, and 6–10 was high risk. The Joanna Briggs Institute (JBI) Appraisal Tool was used to assess the quality of included studies. JBI critical appraisal checklist is one of the appraisal tools that can assess the different types of articles.
Data synthesis and analysis
Meta-analysis was performed using the comprehensive meta-analysis version 2 (CMA2). The pooled frequency of each outcome was estimated using a random-effects model or a fixed-effects model based on heterogeneity between studies. It was expressed as pooled frequencies with a 95% confidence interval (CI). The I2 test was used to measure heterogeneity between studies; I2 > 50% was considered an increase in heterogeneity. A 2-tailed P < 0.05 was considered statistically significant. Continuous data were presented as means (standard deviations) or medians with interquartile ranges (IQRs). All descriptive analyzes were calculated using GraphPad Prism version 9 (version 9, GraphPad Inc).
Results
Selection and characteristics of the articles
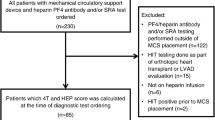
Based on the established search strategy, we found 575 articles in the medical search engines, including 166 articles from PubMed, 265 from Scopus, and 144 from the Web of Science database. After we removed duplicate articles, 333 articles remained. Articles were then screened by title and abstract, and 85 articles remained at this stage. After reviewing the full texts of the remaining articles using the criteria, 37 articles were finally selected. In addition, two articles were collected by reviewing the bibliography of the screened articles. Thus, 39 articles were finally included in the study (Table 1), of which 13 studies were quantitatively analyzed. The flowchart of the study screening process is shown in Fig. 1. All included studies were published between May 2020 and July 2022.
Patients with HIT and COVID-19
Review of the publication date of studies on HIT in patients with COVID-19 has shown that this topic soon attracted attention. The first paper was published in May 2020, about five months after the disease outbreak in China. Various types of studies have been conducted to detect HIT in patients with COVID-19, including 23 case reports, 11 retrospective cohort studies, 4 prospective cohort studies, and 1 randomized controlled trial (up to the time of our search). Among the 39 studies reviewed, various laboratory methods were used to screen patients for the detection of HIT, including ELISA 16 times, LITA 4 times, CTA 4 times, both ELISA and PaGI 1 time, and 14 studies did not report the method used in their surveys. To confirm HIT in patients, various laboratory methods were used, including SRA (in 11 studies), HIPA (in 4 studies), both HIPA and SRA (in 1 study), and 23 studies did not mention the methods used (Table 1). The clinical and laboratory characteristics of patients with COVID-19 and confirmed HIT were extracted from the 39 articles included in the study. Review of the geographic location of the published studies revealed that 16 studies were published from the United States, 15 studies from Europe, 6 from East Asia, 1 from Africa, and 1 from West Asia.
Among 39 articles, 11,334 patients with concomitant COVID-19 and HIT have reported. Among them, HIT was merely confirmed in 123 patients. Our mined data revealed that the complete data of only 37 patients exist; the clinical and laboratory characteristics of patients with COVID-19 and confirmed HIT have shown in Table 2. Of the 37 patients with COVID-19 and confirmed HIT, 30 patients (81%) were treated in the intensive care unit or had severe COVID-19. Of the 37 studies, the type of heparin administered was reported in 33 studies. The most commonly used anticoagulants were UFH in 22 cases (59.4%), LMWH and then UFH in 6 cases (16.2%), LMWH in 5 cases (13.5%), and no information was provided in 4 cases. After the diagnosis HIT, heparin was discontinued and alternative anticoagulants such as argatroban, apixaban, fondaparinux, bivalirudin, danaparoid, and rivaroxaban were used. The most frequently used alternative anticoagulants after confirmation from HIT were argatroban in 15 cases, apixaban in 4 cases, danaparoid in 4 cases, fondaparinux in 4 cases, bivalirudin in 3 cases, rivaroxaban in 1 case, and argatroban with bivalirudin in 1 case. The outcome of patients was 26 alive (70.2%), 5 in limbo (13.5%), 5 dead (13.5%), and 1 (2.7%) with unknown outcome (Table 2). There were 11,311 patients with COVID-19, with HIT confirmed in 37 of them. The median age of the 37 patients was 62 (49.5–65) years, with a sex ratio of 28 men (75.6%) and nine women (24.3%). In addition, the median platelet count before treatment was 237(176–290) x 103/µl and the median nadir platelet count was 52 (31–90.5) x 103/µl, showing a significant decrease in platelet count in the patients with HIT. In addition, the median value of the 4T score in the patients was 6 (4–6) (Table 3).
Frequency of HIT in patients with COVID-19
Thirty-nine studies examined the frequency of HIT in the population of patients with COVID-19, of which 13 had sufficient data to be included in the meta-analysis. The pooled frequency rate of suspected cases with HIT in 13 studies with 11,241 patients was 1.7% (95% CI, 0.6-4.9%; I2 95.24%) with a random-effects model. On the basis of the population studied, studies were divided into 3 subgroups: severe patients requiring support by extracorporeal membrane oxygenation (ECM subgroup), patients with suspected HIT (HIT suspected subgroup), and hospitalized patients (hospitalized subgroup). The frequency of HIT in the ECMO subgroup of 268 patients was 8.2% (95% CI, 6.5-14%; I2 36.14%) by the random-effects model, also one study with 86 patients reported the frequency of HIT in patients with HIT-suspected 8.1% (95% CI, 3.9-16.1%). and in the hospitalization subgroup with 10,887 patients, the frequency of HIT was 0.8% (95% CI, 0.2-2.7%; I2 95.91%) by the random effect model. Heterogeneity between subgroups was significantly different (p: 0.006) (Fig. 2). In order to investigate the publication bias between articles, used Begg’s Funnel plot and Egger’s test (Fig. 3). The Begg’s test and Egger’s test showed that there was no stable evidence of publication bias in the meta-analysis (P: 0.2 and P: 0.45 respectively). In order to measure the effect of each study on the analysis, each article was omitted from the analysis to quantify the frequency of HIT. We did not notice a significant change in the pooled frequency rate before and after extracting each sample. The sensitivity plot is shown in Fig. 4.
Discussion
The current systematic review evaluated information from 39 studies involving 11,334 patients with COVID-19 and HIT. Merely, 13 articles with 11,241 patients included in the meta-analysis. The pooled frequency of antiplatelet factor 4 antibodies (activating and nonactivating antibodies) was 1.7%. Current screening tests for the detection of HIT are based on the detection of antibodies (immunoassay) that detect both pathogenic and nonpathogenic antibodies to platelet factor 4. Therefore, HIT, a potentially life-threatening immunologic disorder, must be confirmed with a confirmatory heparin-dependent platelet activation assay such as SRA or HIPA. In total, there were 37 patients with HIT and COVID-19 in whom one of the confirmatory tests was performed. We hypothesis that If no confirmatory test is available, the rise in platelets after switching from heparin to non-heparin anticoagulants (argatroban, apixaban, fondaparinux, bivalirudin, danaparoid, and rivaroxaban) may be helpful in establishing the diagnosis HIT. In a similar meta-analysis by Uapraserta N. et al. total of 15 of 19 (79%) confirmed HIT cases demonstrated platelet recovery after heparin substitution with non-heparin anticoagulants [27].
Men with COVID-19 were more susceptible to HIT (75.6%) than women with COVID-19 (28 men vs. 9 women). The more frequent occurrence of HIT in men with COVID-19 has been reported in other studies [28,29,30]. This may be because sex hormones influence SARS-CoV-2 penetration, priming, inflammatory process, and susceptibility to thrombosis. It seems that women are better protected against COVID-19 because of some factors, including more effective immune response and lower systemic inflammation [31]. This study showed that 67.5% of patients with HIT and COVID-19 were still alive. This may be due to several factors, including switching to a different anticoagulant, which leads to an increase in platelet count and prevents platelet activation.
Most publications on HIT in COVID-19 are from Western countries. This indicates that the topic of HIT may be neglected or receive little attention in developing countries. Since the mortality rate of HIT is considerable, clinicians concerned with the treatment of patients with COVID-19 in developing countries should take this into account.
Of the 37 confirmed cases with HIT and COVID-19, most patients (30 cases) were hospitalized in the intensive care unit or had severe COVID-19 (81%). This may be due to the tremendous release of cytokines and severe viral sepsis in COVID-19. This indicates that patients with severe COVID-19 are more susceptible to challenge with HIT compared with mild COVID-19. In addition, analysis of the HIT rate in the subgroups showed that HIT occurred more frequently in the HIT suspect group and then in the ECMO group of patients with COVID-19. This reflects that clinical suspicion of HIT by observing a decrease in platelet count is a robust sign of HIT that needs to be confirmed by rapid calculation of the 4Ts score and performance of a confirmatory laboratory test (33). Meanwhile, patients with severe COVID-19 who require oxygen supplementation therapy (which is a symptom of severe COVID-19) are vulnerable to HIT. Therefore, clinicians should keep an eye on the occurrence of HIT in patients with COVID-19 in the ICU. Reviewing the literature, we were able to find a similar meta-analysis across 7 studies involving 5849 patients [32]. The pooled incidence rate of HIT in this meta-analysis was 0.8%. The higher rate of HIT in our study may be due to the fact that we captured a larger number of papers and higher included patients, that HIT has received more attention in after primary reports, and that clinicians are paying more attention to HIT. In addition, the patients included in this meta-analysis had COVID-19, which is associated with a higher risk of thrombosis [15, 33, 34]. This may be attributed to the fact that patients with COVID-19 and HIT have a dual risk of thrombosis.
This study encountered some limitations. There were many cases with HIT, which were not confirmed by HIPA or SRA, while they were positive by screening tests. This may be one of the main causes of bias, reducing the true incidence of HIT. In addition, there were many case reports with incomplete information that did not meet the required criteria to be included in the meta-analysis. Dose of heparin was an interesting finding, but merely few studies have mentioned to it in their reports. This may be because thrombocytopenia is a multifactorial disease, so this complication may not be recognized and subsequently underreported in the medical literature.
Future studies are needed to investigate the impact of HIT on the morbidity and mortality of COVID-19. Another topic of interest in these patients is work on nonpathogenic anti-factor 4 platelet antibodies to show whether these patients are susceptible to developing pathologic antibodies to platelets in future. As for the widespread use of heparin and heparinoids in patients with COVID-19, the bottom line seems to be to keep an eye on thrombocytopenia and HIT in patients with COVID-19.
References
Boshkov LK et al (1993) Heparin-induced thrombocytopenia and thrombosis: clinical and laboratory studies. Br J Haematol 84(2):322–328
Fathi M (2018) Heparin-induced thrombocytopenia (HIT): identification and treatment pathways. Global cardiology science & practice. 2018(2)
Warkentin TE (1998) Clinical presentation of heparin-induced thrombocytopenia. in Seminars in hematology.
Warkentin TE et al (2013) Heparin-induced thrombocytopenia in medical surgical critical illness. Chest 144(3):848–858
Warkentin TE et al (2009) Studies of the immune response in heparin-induced thrombocytopenia blood. J Am Soc Hematol 113(20):4963–4969
Warkentin TE et al (1994) Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia.
Newman PM, Chong BH (2000) Heparin-induced thrombocytopenia: new evidence for the dynamic binding of purified anti-PF4–heparin antibodies to platelets and the resultant platelet activation. Blood The Journal of the American Society of Hematology 96(1):182–187
Kanaan AO, Al-Homsi AS (2009) Heparin-induced thrombocytopenia: pathophysiology, diagnosis, and review of pharmacotherapy. J Pharm Pract 22(2):149–157
Warkentin TE et al (2008) Treatment and prevention of heparin-induced thrombocytopenia: american college of chest Physicians evidence-based clinical practice guidelines. Chest 133(6):340S–380S
Warkentin TE, Chong BH, Greinacher A (1998) Heparin-induced thrombocytopenia: towards consensus. Thromb Haemost 59(01):1–7
Mansouritorghabeh H (2015) Clinical and laboratory approaches to hemophilia A. Iran J Med Sci 40(3):194
Macera M et al (2020) Clinical presentation of COVID-19: case series and review of the literature. Int J Environ Res Public Health 17(14):5062
Wang D et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069
Huang C et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet 395(10223):497–506
Rostami M, Mansouritorghabeh H (2020) D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol 13(11):1265–1275
Mehrdad R, Zahra K, Mansouritorghabeh H (2021) Hemostatic system (Fibrinogen level, D-Dimer, and FDP) in severe and Non-Severe patients With COVID-19: a systematic review and meta-Analysis Clinical and Applied Thrombosis/Hemostasis, 27: p. 10760296211010973
Rostami M, Mansouritorghabeh H, Parsa-Kondelaji M (2021) High levels of Von Willebrand factor markers in COVID-19: a systematic review and meta-analysis.Clinical and Experimental Medicine, : p.1–11
Al-Ani F, Chehade S, Lazo-Langner A (2020) Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res 192:152–160
Hippensteel JA et al (2020) Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiology-Lung Cell Mol Physiol 319(2):L211–L217
Shi C et al (2021) Comprehensive landscape of heparin therapy for COVID-19. Carbohydr Polym 254:117232
ATTACC A-a, Investigators R-C (2021) Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med 385(9):790–802
Cook D et al (2005) Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med 33(7):1565–1571
Ribic C et al (2009) Low-molecular-weight heparin thromboprophylaxis in medical-surgical critically ill patients: a systematic review. J Crit Care 24(2):197–205
Geerts WH et al (2004) Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and thrombolytic therapy. Chest 126(3):338S–400S
Kurtoğlu M et al (2003) Venous thromboembolism prophylaxis with low molecular weight heparins in polytraumatized patients in intensive care unit (extended serie) Ulusal Travma ve Acil Cerrahi Dergisi = turkish. J Trauma Emerg Surgery: TJTES 9(1):37–44
Sholzberg M et al (2021) Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial.bmj,375
Uaprasert N et al (2021) Heparin-induced thrombocytopenia in patients with COVID-19: a systematic review and meta-analysis. Blood Adv 5(21):4521–4534
Daviet F et al (2020) Heparin-Induced Thrombocytopenia in severe COVID-19. Circulation 142(19):1875–1877
Lingamaneni P et al (2020) Heparin-induced thrombocytopenia in COVID-19. J Investig Med High Impact Case Rep 8:2324709620944091
Riker RR et al (2020) Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. 4(5):936–941
Pivonello R et al (2021) Sex disparities in COVID-19 severity and outcome: are men weaker or women stronger? Neuroendocrinology 111(11):1066–1085
Uaprasert N, Tangcheewinsirikul N (2021) Heparin-induced thrombocytopenia in patients with COVID-19: a systematic review and meta-analysis. 5(21):4521–4534
Rostami M, Mansouritorghabeh H (2022) Trend of fluctuations of antithrombin in plasma of patients with COVID-19: a meta-analysis and systematic review. 15(8):747–755
Rostami M, Khoshnegah Z, Mansouritorghabeh H (2021) Hemostatic System (Fibrinogen Level, D-Dimer, and FDP) in Severe and Non-Severe Patients With COVID-19: A Systematic Review and Meta-Analysis 27: p. 10760296211010973
Mohammed HS et al (2022) Accelerated heparin-induced thrombocytopenia in a COVID-19 patient; a case report with literature review.Annals of Medicine and Surgery,78
Siddiqui NA et al (2021) Acute Limb Ischemia complicated by Heparin-Induced Thrombocytopenia in an asymptomatic COVID-19 patient. Cureus 13(7):e16162
Ogawa Y et al (2020) Argatroban therapy for heparin-induced thrombocytopenia in a patient with coronavirus disease 2019. J Thromb Thrombolysis 50(4):1012–1014
Ionescu F et al (2021) Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol 106(2):165–174
Julian K, Bucher D, Jain R (2021) Autoimmune heparin-induced thrombocytopenia: a rare manifestation of COVID-19.BMJ Case Reports, 14(5)
Sasaki K et al (2022) A case of severe COVID-19 with pulmonary thromboembolism related to heparin-induced thrombocytopenia during prophylactic anticoagulation therapy. J Infect Chemother 28(8):1208–1211
Ardiana M et al (2021) Case Report: heparin-induced thrombocytopenia during COVID-19 outbreak: the importance of scoring system in differentiating with sepsis-induced coagulopathy. F1000Research:10
May JE, Siniard RC, Marques M (2020) The challenges of diagnosing heparin-induced thrombocytopenia in patients with COVID-19 Research and Practice in Thrombosis and Haemostasis, 4(6): p. 1066–1067
Delrue M et al (2021) Contrast between prevalence of HIT antibodies and confirmed HIT in hospitalized COVID-19 patients: a prospective study with clinical implications. Thromb Haemost 121(07):971–975
Patell R et al (2020) Heparin induced thrombocytopenia antibodies in Covid-19. Am J Hematol 95(10):E295–E296
Warrior S et al (2020) Heparin Induced Thrombocytopenia in patients with COVID-19. Blood 136:3
Sartori M, Cosmi B (2021) Heparin-induced thrombocytopenia and COVID-19. Hematol Rep 13(1):8857
Huang CT et al (2020) Heparin-induced thrombocytopenia and thrombosis in a patient with Covid-19. Thromb Res 196:11–14
Lingamaneni P et al (2020) Heparin-Induced Thrombocytopenia in COVID-19Journal of Investigative Medicine High Impact Case Reports,8
Bidar F et al (2021) Heparin-induced thrombocytopenia in COVID-19 patients with severe acute respiratory distress syndrome requiring extracorporeal membrane oxygenation: two case reports. J Artif Organs 24(2):277–281
Riker RR et al (2020) Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res Pract Thromb Haemostasis 4(5):936–941
Preti PS et al (2021) Increased prevalence of heparin induced thrombocytopenia in COVID-19 patients. Thromb Res 203:33–35
Madala S, Krzyzak M, Dehghani S (2021) Is COVID-19 an independent risk factor for Heparin-Induced Thrombocytopenia? Cureus 13(2):e13425
Soliman S, Ghaly M (2022) Ischemic stroke and bilateral pulmonary embolism in COVID-19: COVID-Associated Coagulopathy or Heparin-Induced Thrombocytopenia. J Hematol 11(1):40–44
Murakami Y et al (2022) Ischemic stroke due to heparin-induced Thrombocytopenia during severe COVID-19 infection. Intern Med
Lazaro-Garcia A et al (2022) A journey through anticoagulant therapies in the treatment of left ventricular thrombus in post-COVID-19 heparin-induced thrombocytopenia: a case report. Hematology 27(1):318–321
Shiuan E et al (2022) Limb ischemia due to spontaneous heparin-induced thrombocytopenia as the primary presentation of acute COVID-19 infection.Journal of Thrombosis and Thrombolysis, : p.5
Zyani A et al (2021) A rare case of intracerebral hemorrhage complicating heparin-induced thrombocytopenia in a COVID-19 patient. Annals of Medicine and Surgery, p 72
Turshudzhyan A (2020) SARS-CoV2 induced pulmonary embolism and complications from anticoagulation.Respiratory Medicine Case Reports,31
Tran M et al (2020) SARS-CoV-2 and pulmonary embolism: who stole the platelets? Thromb J 18(1):4
Phan XT et al (2020) Suspected heparin-induced thrombocytopenia in a COVID-19 patient on extracorporeal membrane oxygenation support: a case report. Thromb J 18(1):37
Benge EJ, McWhorter Y (2022) Triple threat: bilateral renal artery thrombosis and heparin induced thrombocytopenia in a patient with COVID-19, a case report.Journal of Emergency and Critical Care Medicine,6
Brodard J et al (2021) COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J Thromb Haemost 19(5):1294–1298
Pascreau T et al (2021) The high frequency of anti-PF4/heparin antibodies in patients with COVID-19 is neither related to heparin treatment or to an increased incidence of thrombosis. Clin Chem Lab Med 59(11):E405–E408
Arachchillage DJ et al (2022) Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: a multicentre observational study. Br J Haematol 196(3):566–576
Daviet F et al (2021) Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort.Annals of Intensive Care, 11(1)
Tabatabai A et al (2021) Mortality Risk Assessment in COVID-19 venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 112(6):1983–1989
Lonati PA et al (2022) Production of anti-PF4 antibodies in antiphospholipid antibody-positive patients is not affected by COVID-19 vaccination. Rmd Open 8(1):5
Durak K et al (2021) Thromboembolic and bleeding events in COVID-19 patients receiving extracorporeal membrane oxygenation. Thorac Cardiovasc Surgeon 69(6):526–536
Al-Samkari H et al (2021) Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with covid-19. Ann Intern Med 174(5):622–632
Parzy G et al (2020) Venous thromboembolism events following venovenous extracorporeal membrane oxygenation for severe Acute Respiratory Syndrome Coronavirus 2 based on CT scans. Crit Care Med 48(10):e971–e975
Helms J et al (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46(6):1089–1098
Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19.New England Journal of Medicine, 385(9): p.790–802
Acknowledgements
The authors would like to thank from Vice Chancellor of Research in Mashhad University of Medical Sciences for approving and granting this study.
Funding
Mashhad University of Medical Sciences grant number 4001161.
Author information
Authors and Affiliations
Contributions
M.R. was involved in database searching, screening abstracts and full texts, data extraction, quality assessment, conducting meta-analysis, interpreting data, writing the first draft of the manuscript, and finalizing the manuscript. H.M. was involved in the conception, database search, screening of abstracts and full texts, writing the first draft of the manuscript, and finalizing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Mashhad University of Medical Sciences.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rostami, M., Mansouritorghabeh, H. Significance of heparin induced thrombocytopenia (HIT) in COVID-19: a systematic review and meta-analysis. J Thromb Thrombolysis 56, 241–252 (2023). https://doi.org/10.1007/s11239-023-02827-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02827-5