Abstract
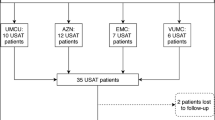
The optimal dose and duration of tissue plasminogen activator (tPA) administered with ultrasound-facilitated catheter-directed thrombolysis (USCDT) to patients with acute PE remains to be determined. Our institution recently adopted a shorter duration (4 h) of USCDT and lower dosing strategy (tPA 1 mg/h) based on data from the OPTALYSE PE Trial. The purpose was to evaluate the implementation at our institution of shorter duration (4 h) of USCDT and lower dosing strategy (tPA 1 mg/h) as outlined by OPTALYSE PE Trial. This was a retrospective, single-center, observational study included patients from 01/01/2017 to 12/31/2018 in a large, academic medical center. Group 1 represented patients who underwent USCDT prior to 01/18/18. Group 2 represented patients who underwent USCDT after 01/18/18 and received 4 h of USCDT and tPA 1 mg/h/catheter. The primary outcome was intensive care unit (ICU) length of stay (LOS). Secondary outcomes were the proportion of patients experiencing a composite of major adverse events (death, recurrent PE, major bleeding, or stroke), change in right ventricle size/function and pulmonary artery pressures, need for mechanical respiratory or hemodynamic support, hospital LOS and drug cost. A total of 31 patients were included in the study: twenty patients in Group 1 and eleven patients in Group 2. Median ICU LOS was 3.5 days in Group 1 and 1 day in Group 2. Group 2 had reduced MACE, requirement for mechanical respiratory or hemodynamic support, hospital LOS, drug costs and adverse events. Implementation of a shorter duration of USCDT and lower dosing strategy for tPA in patients with acute PE may be one strategy to reduce the total ICU LOS and costs associated with care.
Similar content being viewed by others
References
The surgeon general’s call to action to prevent deep vein thrombosis and pulmonary embolism (2008) Rockville (MD)
Kearon C et al (2012) Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141(2):e419S–e496S
Meyer G et al (2014) Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 370(15):1402–1411
Braaten JV, Goss RA, Francis CW (1997) Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost 78(01):1063–1068
Kucher N et al (2014) Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 129(4):479–486
Piazza G et al (2015) A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 8(10):1382–1392
Tapson VF et al (2018) A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv 11(14):1401–1410
Rivera-Lebron B et al (2019) Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT Consortium. Clin Appl Thromb Hemost 25:1076029619853037
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
These individuals have the following to disclose concerning possible financial or personal relationships with commercial entities (or their competitors) that may be referenced in this presentation: Nathaniel B. Wayne, PharmD: Nothing to disclose, George A. Davis, PharmD: Co-Investigator—PE Knockout Study (funding by BTG EKOS®), Tracy E. Macaulay, PharmD: Nothing to disclose, Craig J. Beavers, PharmD: Nothing to disclose, Adrian W. Messerli, MD: Primary Investigator—PE Knockout Study (funding by BTG EKOS®), Adam Dugan, MS: nothing to disclose, Susan S. Smyth, MD, PhD: Nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wayne, N.B., Davis, G.A., Macaulay, T.E. et al. Reduced dose thrombolysis with ultrasound-facilitated catheter-directed administration for acute pulmonary embolism reduces length of stay. J Thromb Thrombolysis 49, 540–544 (2020). https://doi.org/10.1007/s11239-019-02009-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-02009-2