Abstract
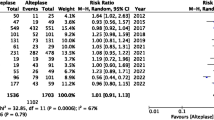
Tenecteplase is a genetically mutated variant of alteplase with superior pharmacodynamic and pharmacokinetic properties. However, its efficacy and safety in acute ischemic strokes are limited. Hence, we conducted a study to evaluate the efficacy and safety of tenecteplase compared with alteplase in acute ischemic stroke. Electronic databases were searched for randomized clinical trials (RCTs) comparing tenecteplase with alteplase in acute ischemic stroke patients eligible for thrombolysis. We evaluated various efficacy and safety outcomes using random-effects models for both pairwise and Bayesian network meta-analyses along with meta-regression analyses. We included 5 RCTs with a total of 1585 patients. Compared with alteplase, tenecteplase treatment was associated with significantly greater complete recanalization (odd ratio [OR] 2.01; 95% confidence interval [CI] 1.04–3.87; p = 0.04) and early neurological improvement (OR 1.43; 95% CI 1.01–2.03; p = 0.05). There were no differences between the two thrombolytics in terms of excellent recovery (modified Rankin Scale [mRS] 0–1; OR 1.17; 95% CI 0.95–1.44; p = 0.13), functional independence (mRS 0–2; OR 1.24; 95% CI 0.78–1.98), poor recovery (mRS 4–6; OR 0.78; 95% CI 0.49–1.25; p = 0.31), complete/partial recanalization (OR 1.51; 95% CI 0.70–3.26; p = 0.30), any intracerebral hemorrhage (OR 0.81; 95% CI 0.56–1.17; p = 0.26), symptomatic intracerebral hemorrhage (OR 0.98; 95% CI 0.52–1.83; p = 0.94), or mortality (OR 0.83; 95% CI 0.54–1.26; p = 0.38). In network meta-analysis, there were better efficacy and imaging-based outcomes with tenecteplase 0.25 mg/kg without increased risk of safety outcomes. Our results demonstrate that in acute ischemic stroke, thrombolysis with tenecteplase is at least as effective and safe as alteplase.


Similar content being viewed by others
References
Powers WJ, Rabinstein AA, Ackerson T et al (2018) 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3):e46–e99
Bhatia R, Hill MD, Shobha N et al (2010) Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41:2254–2258
Lees K, Bluhmki E, von Kummer R et al (2010) Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375:1695–1703
Whiteley WN, Emberson J, Lees KR et al (2016) Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 15:925–933
Keyt BA, Paoni NF, Refino CJ et al (1994) A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci USA 91:3670–3674
Behrouz R (2014) Intravenous tenecteplase in acute ischemic stroke: An updated review. J Neurol 261:1069–1072
Thomas GR, Thibodeaux H, Errett CJ et al (1994) A long-half-life and fibrin-specific form of tissue plasminogen activator in rabbit models of embolic stroke and peripheral bleeding. Stroke 25:2072–2079
Marshall RS (2015) Progress in intravenous thrombolytic therapy for acute stroke. JAMA Neurol 72:928–934
O’Gara PT, Kushner FG, Ascheim DD et al (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127:e362–e425
Ibanez B, James S, Agewall S et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Socie. Eur Hear J 39:119–177
Van De Werf F, Adgey J, Ardissino D et al (1999) Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet 354:716–722
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1–9
Campbell BCV, Mitchell PJ, Churilov L et al (2018) Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 378:1573–1582
Logallo N, Novotny V, Assmus J et al (2017) Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 16:781–788
Huang X, Cheripelli BK, Lloyd SM et al (2015) Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol 14:368–376
Parsons M, Spratt N, Bivard A et al (2012) A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 366:1099–1107
Haley EC, Thompson JLP, Grotta JC et al (2010) Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke 41:707–711
Demaerschalk BM, Kleindorfer DO, Adeoye OM et al (2016) Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 47(2):581–641
Tanswell P, Modi N, Combs D, Danays T (2002) Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 41:1229–1245
Logallo N, Kvistad CE, Thomassen L (2015) Therapeutic potential of tenecteplase in the management of acute ischemic stroke. CNS Drugs 29:811–818
Coutts SB, Dubuc V, Mandzia J et al (2015) Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke 46:769–774
Bivard A, Huang X, Levi CR et al (2017) Tenecteplase in ischemic stroke offers improved recanalization: analysis of 2 trials. Neurology 89:62–67
Zang Y, Hou J, Wang L (2016) Therapeutic effect of tenecteplase on treatment of cerebral arterial thrombosis: a meta-analysis. Eur Rev Med Pharmacol Sci 20:4369–4379
Huang X, MacIsaac R, Thompson JLP et al (2016) Tenecteplase versus alteplase in stroke thrombolysis: An individual patient data meta-analysis of randomized controlled trials. Int J Stroke 11:534–543
Haley EC, Lyden PD, Johnston KC, Hemmen TM (2005) A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke 36:607–612
Armstrong PW, Gershlick AH, Goldstein P et al (2013) Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med 368:1379–1387
Bhatt DL (2013) Timely PCI for STEMI—still the treatment of choice. N Engl J Med 368:1446–1447
Lees KR, Emberson J, Blackwell L et al (2016) Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke 47:2373–2379
Sun C-HJ, Bhatt DL, Nogueira RG, Gupta R (2014) Endovascular therapy for stroke: getting to the “heart” of the matter. Circulation 129:1152–1160
Goyal M, Demchuk AM, Menon BK et al (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372:1019–1030
Schwamm LH (2015) Breaking up is hard to do: tenecteplase in acute stroke. Lancet Neurol 14:343–345
Hankey GJ (2017) Stroke. Lancet 389:641–654
Baird AE (2018) Paving the way for improved treatment of acute stroke with tenecteplase. N Engl J Med 378:1635–1636
Acknowledgements
We would like to thank Katherine Negele, editorial assistant, research department, Hurley Medical Center, for assistance with manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Mustafa Hassan has received a research grant from Abbott. Dr. Mohammed Al Qasmi is on speaker bureau for Genentech. Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. The remaining authors report no relationships that could be construed as a conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kheiri, B., Osman, M., Abdalla, A. et al. Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolysis 46, 440–450 (2018). https://doi.org/10.1007/s11239-018-1721-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1721-3