Abstract
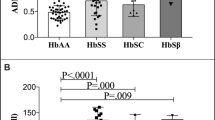
Sickle cell disease (SCD) is characterised by abnormal coagulopathy and angiogenesis although their relationships in two common genotypes, homozygous (HbSS) SCD and sickle-haemoglobin C disease (HbSC), are unexplored. We measured markers of platelet activation (soluble P-selectin [sP-selectin]), fibrinolysis (D-dimer) and angiogenesis (vascular endothelial growth factor [VEGF]) in 27 HbSS patients, 37 HbSC patients and in 42 age and race matched subjects with normal haemoglobin (AA). sP-selectin (P = 0.025) and D-dimers (P < 0.001) were higher in HbSS than in HbSC but there was no difference in VEGF. In HbSC, sPselectin correlated with VEGF (P = 0.012) and D-dimers (P = 0.021). There were no significant correlations in health or in HbSS. Platelet and coagulation activation, but not angiogenic activity, is elevated in HbSS disease compared to the clinically milder HbSC genotype. The correlation between sP-selectin and VEGF in SCD and HbSC disease is consistent with the view that VEGF is released from platelets during in vivo activation.
Similar content being viewed by others
References
Embury SH, Hebbel RP, Steinberg MH, Mohandas N (1994) Pathogenesis of vaso-occlusion. In: Embury SD, Hebbel RP, Mohandas N, Steinberg MH (eds) Sickle cell disease: basic principles and clinical practice. Raven Press, New York, pp 703–724
Francis RB Jr (1991) Platelets, coagulation, and fibrinolysis in sickle cell disease: their possible role in vascular occlusion. Blood Coagul Fibrinolysis 2:341–353
Stuart MJ, Setty BN (2001). Hemostatic alterations in sickle cell disease: relationships to disease pathophysiology. Pediatr Pathol Mol Med 20:27–46
Ataga KI, Orringer EP (2003) Hypercoagulability in sickle cell disease: a curious paradox. Am J Med 115:721–728
Westerman MP, Green D, Gilman-Sachs A, Beaman K, Freels S, Boggio L, Allen S, Zuckerman L, Schlegel R, Williamson P (1999) Antiphospholipid antibodies, proteins C and S, and coagulation changes in sickle cell disease. J Lab Clin Med 134:352–362
Tomer A, Harker LA, Kasey S, Eckman JR (2001) Thrombogenesis in sickle cell disease. J Lab Clin Med 137:398–407
Setty BN, Chen D, O’Neal P, Littrell JB, Grossman MH, Stuart MJ (1998) Eicosanoids in sickle cell disease: potential relevance of 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid to the pathophysiology of vaso-occlusion. J Lab Clin Med 131:344–353
Blann AD, Marwah S, Serjeant G, Bareford D, Wright J (2003) Platelet activation and endothelial cell dysfunction in sickle cell disease is unrelated to reduced antioxidant capacity. Blood Coagul Fibrinolysis 14:255–259
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Solovey A, Gui L, Ramakrishnan S, Steinberg MH, Hebbel RP (1999) Sickle cell anemia as a possible state of enhanced anti-apoptotic tone: survival effect of vascular endothelial growth factor on circulating and unanchored endothelial cells. Blood 93:3824–3830
Marti HH, Risau W (1999) Angiogenesis in ischemic disease. Thromb Haemost 82(Suppl 1):44–52
Mohle R, Green D, Moore MA, Nachman RL, Rafii S (1997) Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A 94:663–668
Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ (1998) Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer 77:956–964
Ballas SK, Lewis CN, Noone AM, Krasnow SH, Kamarulzaman E, Burka ER (1982) Clinical, hematological, and biochemical features of Hb SC disease. Am J Hematol 13:37–51
Hagger D, Wolff S, Owen J, Samson D (1995) Changes in coagulation and fibrinolysis in patients with sickle cell disease compared with healthy black controls. Blood Coagul Fibrinolysis 6:93–99
Singhal A, Doherty JF, Raynes JG, McAdam KPWJ, Thomas PW, Serjeant BE, Serjeant GR (1993) Is there an acute phase response in steady-state sickle cell disease? Lancet 341:651–653
Blann AD, Lip GYH, Beevers DG, McCollum CN (1997) Soluble P-selectin in atherosclerosis: a comparison with endothelial cell and platelet markers. Thromb Haemostas 78:1077–1080
Fijnheer R, Frijns CJ, Korteweg J et al (1997). The origin of P-selectin in atherosclerosis: a comparison with endothelial cell and platelet markers. Thromb Haemostas 77:1081–1085
Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM (1997) Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res 3:2187–2190
Vermeulen PB, Salven P, Benoy I, Gasparini G, Dirix LY (1999) Blood platelets and serum VEGF in cancer patients. Br J Cancer 79:370–373
Zachary I (2001). Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol 280:C1375–C1386
Zachary I, Mathur A, Yla-Herttuala S, Martin J (2000) Vascular protection: a novel nonangiogenic cardiovascular role for vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 20:1512–1520
Minchenko A, Bauer T, Salceda S, Caro J (1994) Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest 71:374–379
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Salgado R, Vermeulen PB, Benoy I, Weytjens R, Huget P, Van Marck E, Dirix LY (1999) Platelet number and interleukin-6 correlate with VEGF but not with bFGF serum levels of advanced cancer patients. Br J Cancer 80:892–897
Bourantas KL, Dalekos GN, Makis A, Chaidos A, Tsiara S, Mavridis A (1998) Acute phase proteins and interleukins in steady state sickle cell disease. Eur J Haematol 61:49–54
Acknowledgements
We thank the staff of the Sickle Cell and Thalassemia Centre, City Hospital for their support of this research. We gratefully acknowledge the funding of the Sandwell and West Birmingham Hospitals NHS Trust Research and Development programme for the Haemostasis Thrombosis and Vascular Biology Unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blann, A.D., Mohan, J.S., Bareford, D. et al. Soluble P-selectin and vascular endothelial growth factor in steady state sickle cell disease: relationship to genotype. J Thromb Thrombolysis 25, 185–189 (2008). https://doi.org/10.1007/s11239-007-0177-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-007-0177-7