Abstract
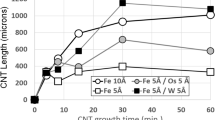
The influence of flow rate and concentration of source of hydrocarbon on the properties of the carbon nanotubes formed in the process of thermal decomposition of ethylene on nickel nanoparticles have been investigated. Changes in the rate of gaseous flow has practically no effect on the specific yield and the mean diameter of the nanotubes. However, an increase in the mean diameter and a decrease in the value of the specific yield occurs with increase in the concentration of ethylene.
Similar content being viewed by others
References
M. Rao, D. Jacques, R. C. Haddon, et al., Appl. Phys. Lett., 76, 3813–3815 (2000).
P. Serp, M. Corrias, and P. Kalck, Appl. Catal. A., 253, 337–358 (2004).
A. P. Ramirez, Bells Labs Tech. J., 10, No. 3, 171–185 (2005).
É. G. Rakov, Usp. Khim., 76, No. 1, 3–26 (2007).
H. Kenji, N. F. Don, M. Kohei, et al., Science, 306, 1362–1364 (2004).
Md. Shajahan, Y. H. Mo, A. K. M. Fazle Kibria, et al., Carbon, 42, 2245–2253 (2004).
A. Morançais, B. Caussat, Y. Khin, et al., Carbon, 45, 624–635 (2007).
V. A. Khavrus’, N. V. Lemesh, S. V. Gordeichuk, et al., Teor. Éksp. Khim., 42, No. 4, 227–230 (2006). [Theor. Experim. Chem., 42, No. 4, 234–238 (2006) (Engl. Transl.).]
J. Lefebvre, R. Antonov, and A. T. Johnson, Phys. A, 67, No. 1, 71–74 (1998).
A. Barreiro, C. Kramberger, M. H. Rümmeli, et al., Carbon, 45, 55–61 (2007).
A. K. M. Fazle Kibria, Y. H. Mo, and K. S. Nahm, Catal. Lett., 71, Nos. 3/4, 229–236 (2001).
A. M. Cassell, J. A. Raymakers, J. Kong, and H. Dai, J. Phys. Chem., 103, 6484–6492 (1999).
R. T. K. Baker, Carbon, 27, No. 3, 315–323 (1989).
R. A. Byyanov and V. V. Chesnokov, Khim. Interes. Ustoich. Razvit., 3, No. 3, 177–186 (1995).
Y. Li, W. Kim, Y. Zhang, et al., J. Phys. Chem. B, 105, 11424–11431 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 44, No. 4, pp. 228–232, July–August, 2008.
Rights and permissions
About this article
Cite this article
Tripol’skii, A.I., Lemesh, N.V., Khavrus’, V.A. et al. Morphology of carbon nanotubes, obtained by decomposition of ethylene on nickel nanoparticles at various rates of flow and concentration of C2H4 . Theor Exp Chem 44, 240–244 (2008). https://doi.org/10.1007/s11237-008-9034-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-008-9034-9