Abstract
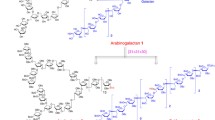
The review considers the known strategies for the synthesis of fragments of arabinogalactan and lipoarabinomannan, polysaccharides that are contained in the cell walls of causative agent of tuberculosis Mycobacterium tuberculosis, as well as other related oligosaccharides containing arabinofuranose residues, using silyl substituents both for the differentiation of hydroxy groups in monosaccharide blocks and as stereodirecting groups during formation of a β-arabinofuranoside bond. In particular, the use of silyl groups (in combination with orthogonal to them acyl groups) allows the stereoselective synthesis of arabinans without the involvement of benzyl groups removable under reducing conditions, which is unacceptable in the presence of fragments sensitive to hydrogenolysis. This significantly simplifies the synthesis of 1,2-cis-linked oligosaccharides containing multiple bonds or azide groups, in particular, in aglycone, which is extremely important for the preparation of neoglycoconjugates both by converting the azide to an amine, followed by covalent binding to a carrier, and by conducting click-chemistry reactions using the 1,3-dipolar cycloaddition of azides to alkynes.
Similar content being viewed by others
References
W. F. Paolo, J. D. Nosanchuk, Lancet Infect. Dis., 2004, 4, 287; DOI: https://doi.org/10.1016/s1473-3099(04)01004-7.
P. D. O. Davies, Ann. Med., 2009, 35, 235; DOI: https://doi.org/10.1080/07853890310005713.
World Health Organization. Tuberculosis (TB); https://www.who.int/tb/en (accessed May 1, 2021).
World Health Organization. Tuberculosis Fact Sheet;https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (accessed May 1, 2021).
P.-H. Tam, T. L. Lowary, in Carbohydrate Chemistry, Eds A. P. Rauter and T. K. Lindhorst, The Royal Society of Chemistry, 2010, 38; DOI: https://doi.org/10.1039/9781849730891-00038.
B. Hamasur, G. Ka, S. B. Svenson, Vaccine, 1999, 17, 2853.
R. B. Zheng, S. A. F. Jegouzo, M. Joe, Y. Bai, H. A. Tran, K. Shen, J. Saupe, L. Xia, M. F. Ahmed, Y. H. Liu, P. S. Patil, A. Tripathi, S. C. Hung, M. E. Taylor, T. L. Lowary, K. Drickamer, ACS Chem. Biol., 2017, 12, 2990; DOI: https://doi.org/10.1021/acschembio.7b00797.
G. L. Burygin, P. I. Abronina, N. M. Podvalnyy, S. A. Staroverov, L. O. Kononov, L. A. Dykman, Beilstein J. Nanotechnol., 2020, 11, 480; DOI: https://doi.org/10.3762/bjnano.11.39.
H.-S. Kim, E. S. M. Ng, R. B. Zheng, R. M. Whittal, D. C. Schriemer, T. L. Lowary, in Carbohydrate-Based Vaccines, Ed. R. Roy, American Chemical Society, 2008, 184.
T. T. Chen, C. Blanc, Y. Y. Liu, E. Ishida, S. Singer, J. Y. Xu, M. Joe, E. R. Jenny-Avital, J. Chan, T. L. Lowary, J. M. Achkar, J. Clin. Invest., 2020, 130, 1808; DOI: https://doi.org/10.1172/jci128459.
Z. H. Li, T. Bavaro, S. Tengattini, R. Bernardini, M. Mattei, F. Annunziata, R. B. Cole, C. P. Zheng, M. Sollogoub, L. Tamborini, M. Terreni, Y. M. Zhang, Eur. J. Med. Chem., 2020, 204, 112578; DOI: https://doi.org/10.1016/j.ejmech.2020.112578.
Y. Chu, J. S. Yang, Carbohydr. Res., 2018, 465, 10; DOI: https://doi.org/10.1016/j.carres.2018.05.009.
L. Wang, S. Feng, S. Wang, H. Li, Z. Guo, G. Gu, J. Org. Chem., 2017, 82, 12085; DOI: https://doi.org/10.1021/acs.joc.7b01817.
J. Gao, G. Liao, L. Wang, Z. Guo, Org. Lett., 2014, 16, 988; DOI: https://doi.org/10.1021/ol4036903.
R. J. Coker, Trop. Med. Int. Health, 2004, 9, 25; DOI: https://doi.org/10.1046/j.1365-3156.2003.01156.x.
M. Tong, C. E. Jacobi, F. M. van de Rijke, S. Kujiper, S. van de Werken, T. L. Lowary, C. H. Hokke, B. J. Appelmelk, N. J. D. Nagelkerke, H. J. Tanke, R. P. M. van Gijlswijk, J. Veuskens, A. H. J. Kolk, A. K. Raap, J. Immunol. Methods, 2005, 301, 154; DOI: https://doi.org/10.1016/j.jim.2005.04.004.
P. I. Abronina, N. M. Podvalnyy, T. M. Mel’nikova, A. I. Zinin, K. G. Fedina, V. V. Kachala, V. I. Torgov, L. O. Kononov, E. A. Panfertsev, E. V. Baranova, V. V. Mochalov, V. I. Dyatlova, S. F. Biketov, Russ. Chem. Bull., 2010, 59, 2333; DOI: https://doi.org/10.1007/s11172-010-0397-4.
A. G. Korolyova-Ushakova, E. V. Baranova, S. G. Ignatov, P. V. Soloviev, N. N. Kondakov, T. M. Mel’nikova, P. I. Abronina, N. M. Podval’nyi, L. O. Kononov, S. F. Biketov, Appl. Biochem. Microbiol., 2019, 55, 696; DOI: https://doi.org/10.1134/S0003683819060097.
H. B. Mereyala, S. Hotha, M. K. Gurjar, Chem Commun., 1998, 685; DOI: https://doi.org/10.1039/a707796c.
A. Ishiwata, H. Akao, Y. Ito, Org. Lett., 2006, 8, 5525; DOI: https://doi.org/10.1021/ol062198j.
M. Joe, Y. Bai, R. C. Nacario, T. L. Lowary, J. Am. Chem. Soc., 2007, 129, 9885; DOI: https://doi.org/10.1021/ja072892+.
K. Sahloul, T. L. Lowary, J. Org. Chem., 2015, 80, 11417; DOI: https://doi.org/10.1021/acs.joc.5b02083.
M. Islam, G. Gayatri, S. Hotha, J. Org. Chem., 2015, 80, 7937; DOI: https://doi.org/10.1021/acs.joc.5600964.
T. L. Lowary, Acc. Chem. Res., 2016, 49, 1379; DOI: https://doi.org/10.1021/acs.accounts.6b00164.
B. Mishra, S. Manmode, R. R. A. Panda, S. Hotha, Eur. J. Org. Chem., 2017, 2017, 4794; DOI: https://doi.org/10.1002/ejoc.201700712.
Y. Wu, D. C. Xiong, S. C. Chen, Y. S. Wang, X. S. Ye, Nat. Commun., 2017, 8, 14851, DOI: https://doi.org/10.1038/ncomms14851; DOI: https://doi.org/10.1038/ncomms14851.
H. Z. Li, J. Ding, C. R. Cheng, Y. Chen, X. Y. Liang, Carbohydr. Res., 2018, 460, 1; DOI: https://doi.org/10.1016/j.carres.2018.02.006.
A. Pardo-Vargas, P. Bharate, M. Delbianco, P. H. Seeberger, Beilstein J. Org. Chem., 2019, 15, 2936; DOI: https://doi.org/10.3762/bjoc.15.288.
L. Han, L. Wang, Z. Guo, J. Carbohydr. Chem., 2019, 38, 335; DOI: https://doi.org/10.1080/07328303.2019.1630840.
K. Liu, L. Wang, Z. Guo, J. Carbohydr. Chem., 2019, 38, 414; DOI: https://doi.org/10.1080/07328303.2019.1630841.
A. Hölemann, B. L. Stocker, P. H. Seeberger, J. Org. Chem., 2006, 71, 8071; DOI: https://doi.org/10.1021/jo061233x.
B. Fraser-Reid, J. Lu, K. N. Jayaprakash, J. C. López, Tetrahedron Asymmetry, 2006, 17, 2449; DOI: https://doi.org/10.1016/j.tetasy.2006.09.008.
A. Ishiwata, Y. Ito, J. Am. Chem. Soc., 2011, 133, 2275; DOI: https://doi.org/10.1021/ja109932t.
V. Behar, S. J. Danishefsky, Angew. Chem., Int. Ed. Engl., 1994, 33, 1468; DOI: https://doi.org/10.1002/anie.199414681.
J. T. Randolph, S. J. Danishefsky, Angew. Chem., Int. Ed. Engl., 1994, 33, 1470; DOI: https://doi.org/10.1002/anie.199414701.
T. Ziegler, in Carbohydrate chemistry, Ed. G.-J. Boons, Blackie Academic & Professional, London—New York, 1998, 21.
J. Lawandi, S. Rocheleau, N. Moitessier, Tetrahedron, 2016, 72, 6283; DOI: https://doi.org/10.1016/j.tet.2016.08.019.
M. Bols, C. M. Pedersen, Beilstein J. Org. Chem., 2017, 13, 93; DOI: https://doi.org/10.3762/bjoc.13.12.
V. Dimakos, M. S. Taylor, Chem. Rev., 2018, 118, 11457; DOI: https://doi.org/10.1021/acs.chemrev.8b00442.
Protective groups: Strategies and Applications in Carbohydrate Chemistry, Ed. S. Vidal, Wiley—VCH Verlag GmbH & Co. KGaA, Weinheim, 2019, 528 pp.
E. J. Corey, A. Venkateswarlu, J. Am. Chem. Soc., 1972, 94, 6190; DOI: https://doi.org/10.1021/ja00772a043.
S. Hanessian, P. Lavallee, Can. J. Chem. Rev. Can. Chim., 1975, 53, 2975; DOI: https://doi.org/10.1139/v75-419.
L. H. Sommer, Stereochemistry, Mechanism and Silicon; an Introduction to the Dynamic Stereochemistry and Reaction Mechanisms of Silicon Centers, McGraw-Hill, New York, 1965, 189 pp.
K. K. Ogilvie, K. L. Sadana, E. A. Thompson, M. A. Quilliam, J. B. Westmore, Tetrahedron Lett., 1974, 15, 2861; DOI: https://doi.org/10.1016/S0040-4039(01)91763-0.
C. Ruecker, Chem. Rev., 1995, 95, 1009; DOI: https://doi.org/10.1021/cr00036a006.
O. Dahlman, P. J. Garegg, H. Mayer, S. Schramek, Acta Chem. Scand. B, 1986, 40, 15; DOI: https://doi.org/10.3891/acta.chem.scand.40b-0015.
A. K. Pathak, V. Pathak, N. Bansal, J. A. Maddry, R. C. Reynolds, Tetrahedron Lett., 2001, 42, 979; DOI: https://doi.org/10.1016/s0040-4039(00)02161-4.
H. Yin, F. W. D’Souza, T. L. Lowary, J. Org. Chem., 2002, 67, 892; DOI: https://doi.org/10.1021/jo010910e.
F. W. D’Souza, J. D. Ayers, P. R. McCarren, T. L. Lowary, J. Am. Chem. Soc., 2000, 122, 1251; DOI: https://doi.org/10.1021/ja9935431.
S. K. Chaudhary, O. Hernandez, Tetrahedron Lett., 1979, 20, 99; DOI: https://doi.org/10.1016/S0040-4039(01)85893-7.
X. M. Zhu, S. Kawatkar, Y. Rao, G. J. Boons, J. Am. Chem. Soc., 2006, 128, 11948; DOI: https://doi.org/10.1021/ja0629817.
T. Ziegler, E. Eckhardt, G. Pantkowski, J. Carbohydr. Chem., 1994, 13, 81; DOI: https://doi.org/10.1080/07328309408009180.
D. Crich, C. M. Pedersen, A. A. Bowers, D. J. Wink, J. Org. Chem., 2007, 72, 1553; DOI: https://doi.org/10.1021/jo061440x.
P. O. Adero, H. Amarasekara, P. Wen, L. Bohe, D. Crich, Chem. Rev., 2018, 118, 8242; DOI: https://doi.org/10.1021/acs.chemrev.8b00083.
P. I. Abronina, N. M. Podvalnyy, S. L. Sedinkin, K. G. Fedina, A. I. Zinin, A. O. Chizhov, V. I. Torgov, L. O. Kononov, Synthesis, 2012, 44, 1219; DOI: https://doi.org/10.1055/s-0031-1290752.
P. Deslongchamps, Stereoelectronic Effects in Organic Chemistry, 1st ed., Pergamon Press, Oxford Oxfordshire—New York, 1983, 375 pp.
A. J. Kirby, The Anomeric Effect and Related Stereoelectronic Effects at Oxygen, Springer—Verlag, Berlin—New York, 1983, 149 pp.
A. J. Kirby, Stereoelectronic Effects, Oxford University Press, Oxford—New York, 1996, 89 pp.
E. Juaristi, G. Cuevas, The Anomeric Effect, CRC Press, Boca Raton, 1995, 229 pp.
U. Ellervik, G. Magnusson, J. Am. Chem. Soc., 1994, 116, 2340; DOI: https://doi.org/10.1021/ja00085a013.
J. B. Houseknecht, T. L. Lowary, C. M. Hadad, J. Phys. Chem. A, 2003, 107, 5763; DOI: https://doi.org/10.1021/jp027716w.
R. U. Lemieux, K. B. Hendriks, R. V. Stick, K. James, J. Am. Chem. Soc., 1975, 97, 4056; DOI: https://doi.org/10.1021/ja00847a032.
T. L. Lowary, Curr. Opin. Chem. Biol., 2003, 7, 749; DOI: https://doi.org/10.1016/j.cbpa.2003.10.005.
C. H. Larsen, B. H. Ridgway, J. T. Shaw, D. M. Smith, K. A. Woerpel, J. Am. Chem. Soc., 2005, 127, 10879; DOI: https://doi.org/10.1021/ja0524043.
M. G. Beaver, T. M. Buscagan, O. Lavinda, K. A. Woerpel, Angew. Chem., Int. Ed., 2016, 55, 1816; DOI: https://doi.org/10.1002/anie.201507806.
Y. Rao, G. J. Boons, Angew. Chem., Int. Ed., 2007, 46, 6148; DOI: https://doi.org/10.1002/anie.200701750.
J. D. C. Codée, L. J. Van Den Bos, R. E. J. N. Litjens, H. S. Overkleeft, C. A. A. Van Boeckel, J. H. Van Boom, G. A. Van Der Marel, Tetrahedron, 2004, 60, 1057; DOI: https://doi.org/10.1016/j.tet.2003.11.084.
B. Fraser-Reid, J. C. Lopez, Top. Curr. Chem., 2011, 301, 1; DOI: https://doi.org/10.1007/128_2010_105.
H. D. Premathilake, A. V. Demchenko, Top. Curr. Chem., 2011, 301, 189; DOI: https://doi.org/10.1007/128_2010_106.
X. Y. Liang, H. C. Bin, J. S. Yang, Org. Lett., 2013, 15, 2834; DOI: https://doi.org/10.1021/ol401166x.
S. Wang, X. Meng, W. Huang, J.-S. Yang, J. Org. Chem., 2014, 79, 10203; DOI: https://doi.org/10.1021/jo5018684.
A. Imamura, Trends Glycosci. Glycotechnol., 2014, 26, 141; DOI: https://doi.org/10.4052/tigg.26.141.
P. I. Abronina, K. G. Fedina, N. M. Podvalnyy, A. I. Zinin, A. O. Chizhov, N. N. Kondakov, V. I. Torgov, L. O. Kononov, Carbohydr. Res., 2014, 396, 25; DOI: https://doi.org/10.1016/j.carres.2014.05.017.
K. G. Fedina, P. I. Abronina, N. M. Podvalnyy, N. N. Kondakov, A. O. Chizhov, V. I. Torgov, L. O. Kononov, Carbohydr. Res., 2012, 357, 62; DOI: https://doi.org/10.1016/j.carres.2012.05.021.
P. I. Abronina, S. L. Sedinkin, N. M. Podvalnyy, K. G. Fedina, A. I. Zinin, V. I. Torgov, L. O. Kononov, Tetrahedron Lett., 2011, 52, 1794; DOI: https://doi.org/10.1016/j.tetlet.2011.02.019.
A. Imamura, T. L. Lowary, Org. Lett., 2010, 12, 3686; DOI: https://doi.org/10.1021/ol101520q.
N. M. Podvalnyy, P. I. Abronina, K. G. Fedina, N. N. Kondakov, A. I. Zinin, A. O. Chizhov, V. I. Torgov, V. V. Kachala, L. O. Kononov, Russ. Chem. Bull., 2015, 64, 1149; DOI: https://doi.org/10.1007/s11172-015-0992-5.
V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596; DOI: https://doi.org/10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4.
M. Meldal, C. W. Tornoe, Chem. Rev., 2008, 108, 2952; DOI: https://doi.org/10.1021/cr0783479.
V. K. Tiwari, B. B. Mishra, K. B. Mishra, N. Mishra, A. S. Singh, X. Chen, Chem. Rev., 2016, 116, 3086; DOI: https://doi.org/10.1021/acs.chemrev.5b00408.
X. P. He, Y. L. Zeng, Y. Zang, J. Li, R. A. Field, G. R. Chen, Carbohydr. Res., 2016, 429, 1; DOI: https://doi.org/10.1016/j.carres.2016.03.022.
V. Poonthiyil, T. K. Lindhorst, V. B. Golovko, A. J. Fairbanks, Beilstein J. Org. Chem., 2018, 14, 11; DOI: https://doi.org/10.3762/bjoc.14.2.
N. M. Podvalnyy, P. I. Abronina, E. L. Zdorovenko, A. O. Chizhov, A. I. Zinin, V. I. Torgov, L. O. Kononov, Russ. Chem. Bull., 2014, 63, 497; DOI: https://doi.org/10.1007/s11172-014-0459-0.
P. I. Abronina, A. I. Zinin, N. N. Malysheva, E. V. Stepanova, A. O. Chizhov, V. I. Torgov, L. O. Kononov, Synlett, 2017, 28, 1608; DOI: https://doi.org/10.1055/s-0036-1589028.
P. I. Abronina, A. I. Zinin, D. A. Romashin, V. V. Tereshina, A. O. Chizhov, L. O. Kononov, Carbohydr. Res., 2018, 464, 28; DOI: https://doi.org/10.1016/j.carres.2018.05.005.
U. Jost, P. I. Abronina, A. I. Zinin, D. Michalik, U. Kragl, N. N. Kondakov, A. O. Chizov, V. I. Torgov, L. O. Kononov, Russ. Chem. Bull., 2018, 67, 2297; DOI: https://doi.org/10.1007/s11172-018-2373-3.
E. V. Stepanova, N. M. Podvalnyy, P. I. Abronina, L. O. Kononov, Synlett, 2018, 29, 2043; DOI: https://doi.org/10.1055/s-0037-1610648.
N. N. Kondakov, M. V. Panova, P. I. Abronina, A. I. Zinin, A. M. Shpirt, L. O. Kononov, Russ. Chem. Bull., 2019, 68, 416; DOI: https://doi.org/10.1007/s11172-019-2402-x.
P. Abronina, A. Zinin, A. Chizhov, L. Kononov, Eur. J. Org. Chem., 2020, 4146; DOI: https://doi.org/10.1002/ejoc.202000520.
N. M. Podvalnyy, A. O. Chizhov, A. I. Zinin, L. O. Kononov, Carbohydr. Res., 2016, 431, 25; DOI: https://doi.org/10.1016/j.carres.2016.05.009.
S.-J. Hou, R. Saksena, P. Kováč, Carbohydr. Res., 2008, 343, 196; DOI: https://doi.org/10.1016/j.carres.2007.10.015.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. M. Nefedov on the occasion of his 90th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 6–29, January, 2022.
This work was financially supported by the Russian Science Foundation (Project No. 21-73-20164).
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Abronina, P.I., Podvalnyy, N.M. & Kononov, L.O. The use of silyl groups in the synthesis of arabinofuranosides. Russ Chem Bull 71, 6–29 (2022). https://doi.org/10.1007/s11172-022-3371-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3371-z