Abstract
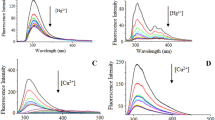
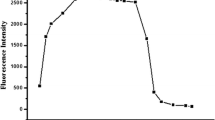
The 4-acetylamino-1,8-naphthalimide derivative containing the N-phenylazadithia-15-crown-5-ether fragment in the N-aryl substituent at the imide nitrogen atom of the naphthalimide core was synthesized, and its cation-dependent spectral properties were studied. The resulting compound in the photoexcited state exhibits low-intensity fluorescence due to the process of electron transfer from the N-aryl group to the naphthalimide residue, which is confirmed by the data of quantum chemical calculations performed using the PM6 method. The binding of Hg2+ in an aqueous acetate buffer solution at pH 6.0 is accompanied by the formation of a 1: 1 metal—ligand complex in which the electron transfer is suppressed leading to fluorescence enhancement. The observed spectral changes were used for the determination of the stability constant K of the complex (logK = 6.51±0.03). The found limit of Hg2+ detection using the synthesized sensor (28 nmol L−1) is fairly close the maximum permissible concentration for mercury in drinking water. The study of the selectivity of complexation showed that the presence of Cu2+, Zn2+, Ni2+, Pb2+ Cd2+, Ca2+, Mg2+, and Fe2+ cations did not impede the determination of Hg2+. The presented results indicate that the synthesized chemosensor is promising as a selective and highly sensitive fluorescent reagent for Hg2+ ions in an aqueous solution.
Similar content being viewed by others
References
Z. Xu, J. Yoon, D. R. Spring, Chem. Soc. Rev., 2010, 39, 1996; DOI: https://doi.org/10.1039/b916287a.
Y. Jeong, J. Yoon, Inorganica Chim. Acta, 2012, 381, 2; DOI: https://doi.org/10.1016/j.ica.2011.09.011.
G. Sivaraman, M. Iniya, T. Anand, N. G. Kotla, O. Sunnapu, S. Singaravadivel, A. Gulyani, D. Chellappa, Coord. Chem. Rev., 2018, 357, 50; DOI: https://doi.org/10.1016/j.ccr.2017.11.020.
M. Harada, B. R. Von, J. Appl. Toxicol., 1995, 15, 483; DOI: https://doi.org/10.1002/jat.2550150610.
H. Harris, I. Pickering, G. George, Science, 2003, 301, 1203; DOI: https://doi.org/10.1126/science.1085941.
E. Martin, R. Weigand, A. Pardo, J. Luminesc., 1996, 68, 157; DOI: https://doi.org/10.1016/0022-2313(96)00008-7.
I. Grabchev, T. Konstantinova, Dyes Pigm., 1997, 33, 197; DOI: https://doi.org/10.1016/S0143-7208(96)00053-8.
P. Panchenko, M. Grin, O. Fedorova, M. Zakharko, D. Pritmov, A. Mironov, A. Arkhipova, Y. Fedorov, G. Jonusauskas, R. Yakubovskaya, N. Morozova, A. Ignatova, A. Feofanov, Phys. Chem. Chem. Phys., 2017, 19, 30195; DOI: https://doi.org/10.1039/C7CP04449F.
H. Lin, Y. Chan, J. Chen, C. Chang, J. Mater. Chem., 2011, 21, 3170; DOI: https://doi.org/10.1039/C0JM02942D.
O. Krasnovskaya, V. Malinnikov, N. Dashkova, V. Gerasimov, I. Grishina, I. Kireev, S. Lavrushkina, P. Panchenko, M. Zakharko, P. Ignatov, O. Fedorova, G. Jonusauskas, D. Skvortsov, S. Kovalev, E. Beloglazkina, N. Zyk, A. Majouga, Bioconj. Chem., 2019, 30, 741; DOI: https://doi.org/10.1021/acs.bioconjchem.8b00885.
M. A. Zakharko, P. A. Panchenko, D. P. Zarezin, V. G. Nenajdenko, D. A. Pritmov, M. A. Grin, A. F. Mironov, O. A. Fedorova, Russ. Chem. Bull., 2020, 69, 1169; DOI: https://doi.org/10.1007/s11172-020-2885-5.
R. Tandon, V. Luxami, H. Kaur, N. Tandon, K. Paul, Chem. Record., 2017, 17, 956; DOI: https://doi.org/10.1002/tcr.201600134.
L. Ingrassia, F. Lefranc, R. Kiss, T. Mijatovic, Curr. Med. Chem., 2009, 16, 1192; DOI: https://doi.org/10.2174/092986709787846659.
X. Zheng, Q. Peng, J. Lin, Y. Wang, J. Zhou, Y. Jiao, Y. Bai, Y. Huang, F. Li, X. Liu, X. Pu, Z. Lu, J. Mater. Chem. C, 2015, 3, 6970; DOI: https://doi.org/10.1039/C5TC00779H.
G. Tu, Q. Zhou, Y. Chen, Y. Geng, L. Wang, D. Ma, X. Jing, F. Wang, Synth. Met., 2005, 152, 233; DOI: https://doi.org/10.1016/j.synthmet.2005.07.236.
S. Yang, J. Liu, Z. Cao, M. Li, Q. Luo, D. Qu, Dyes Pigm., 2018, 148, 341; DOI: https://doi.org/10.1016/j.dyepig.2017.09.040.
A. N. Arkhipova, P. A. Panchenko, Yu. V. Fedorov, O. A. Fedorova, Mendeleev Commun., 2017, 27, 53; DOI: https://doi.org/10.1016/j.mencom.2017.01.016.
O. Fedorova, A. Sergeeva, P. Panchenko, Yu. Fedorov, F. Erko, J. Berthet, S. Delbaere, J. Photochem. Photobiol. A, 2015, 28, 303; DOI: https://doi.org/10.1016/j.jphotochem.2015.02.004.
S. Aderinto, S. Imhanria, Chem. Papers, 2018, 72, 1823; DOI: https://doi.org/10.1007/s11696-018-0411-0.
P. A. Panchenko, O. A. Fedorova, Yu. V. Fedorov, Russ. Chem. Rev., 2014, 83, 155; DOI: https://doi.org/10.1070/RC2014v083n02ABEH004380.
P. A. Panchenko, P. A. Ignatov, M. A. Zakharko, Yu. V. Fedorov, O. A. Fedorova, Mendeleev Commun., 2020, 30, 55; DOI: https://doi.org/10.1016/j.mencom.2020.01.018.
P. Panchenko, Yu. Fedorov, V. Perevalov, G. Jonusauskas, O. Fedorova, J. Phys. Chem. A, 2010, 114, 4118; DOI: https://doi.org/10.1021/jp9103728.
P. A. Panchenko, Yu. V. Fedorov, O. A. Fedorova, B. A. Izmailov, V. A. Vasnev, V. V. Istratov, E. A. Makeeva, M. N. Rumyantseva, A. M. Gaskov, Mendeleev Commun., 2011, 21, 12; DOI: https://doi.org/10.1016/j.mencom.2011.01.005.
A. N. Sergeeva, P. A. Panchenko, Yu. V. Fedorov, O. A. Fedorova, Protec. Met. Phys. Chem. Surf., 2012, 48, 524; DOI: https://doi.org/10.1134/S2070205112050103].
P. Panchenko, Yu. Fedorov, O. Fedorova, G. Jonusauskas, Dyes Pigm., 2013, 98, 347; DOI: https://doi.org/10.1016/j.dyepig.2013.03.008.
P. A. Panchenko, V. V. Park, O. A. Fedorova, Yu. V. Fedorov, E. A. Kataev, Russ. Chem. Bull., 2015, 64, 1871; DOI: https://doi.org/10.1007/s11172-015-1086-0.
P. A. Panchenko, N. V. Leichu, Yu. V. Fedorov, O. A. Fedorova, Macroheterocycles, 2019, 12, 319; DOI: https://doi.org/10.6060/MHC190339p.
P. Panchenko, A. Efremenko, A. Feofanov, M. Ustimova, Yu. Fedorov, O. Fedorova, Sensors, 2021, 21, 470; DOI: https://doi.org/10.3390/S21020470.
P. Panchenko, Yu. Fedorov, O. Fedorova, G. Jonusauskas, Phys. Chem. Chem. Phys., 2015, 17, 22749; DOI: https://doi.org/10.1039/C5CP03510D.
K. Hearn, T. Nalder, R. Cox, H. Maynard, T. Bell, F. Pfeffer, T. Ashton, Chem. Commun., 2017, 53, 12298; DOI: https://doi.org/10.1039/C7CC07922B.
S. L Selector, L. B. Bogdanova, A. V. Shokurov, P. A. Panchenko, O. A. Fedorova, V. V. Arslanov, Macroheterocycles, 2014, 7, 311; DOI: https://doi.org/10.6060/mhc140506s.
J. Stewart, J. Mol. Model., 2007, 13, 1173; DOI: https://doi.org/10.1007/s00894-007-0233-4.
N. J. Turro, V. Ramamurthy, J. C. Scaiano, Modern Molecular Photochemistry of Organic Molecules, New Delhi, Viva Books Private Limited, 2017, 1084 pp.
A. Walcarius, M. Etienne, C. Delacote, Anal. Chim. Acta, 2004, 508, 87; DOI: https://doi.org/10.1016/j.aca.2003.11.055.
H. Loock, P. Wentzell, Sens. Actuators B, 2012, 173, 157; DOI: https://doi.org/10.1016/j.snb.2012.06.071.
G. Chen, W. Chen, Yu. Yen, Ch. Wang, H. Chang, Ch. Chen, Anal. Chem., 2014, 86, 6843. DOI: https://doi.org/10.1021/ac5008688.
P. A. Panchenko, A. S. Polyakova, Y. V. Fedorov, O. A. Fedorova, Mendeleev Commun., 2019, 29, 155; DOI: https://doi.org/10.1016/j.mencom.2019.03.012.
V. A. Rabinovich, Z. Ya. Khavin, Kratkii khimicheskii spravochnik [Brief Chemical Manual], Khimiya, Leningrad, 1977 (in Russian).
R. M. C. Dawson, D. C. Elliott, W. H. Elliott, K. M. Jones, Data for Biochemical Research, Oxford, Clarendon Press, 1986, 580 pp.
C. Renschler, L. Harrah, Anal. Chem., 1983, 55, 798, DOI: https://doi.org/10.1021/ac00255a050.
S. Nad, M. Kumbhakar, H. Pal, Phys. Chem. A, 2003, 107, 4808; DOI: https://doi.org/10.1021/jp021543t.
K. A. Connors, Binding Constants: the Measurement of Molecular Complex Stability, John Wiley & Sons, New York, 1987.
M. T. Beck, I. Nagypál, Chemistry of Complex Equilibria, John Wiley & Sons, New York, 1990.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1939–1945, October, 2021.
This paper does not contain descriptions of studies on animals or humans.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Panchenko, P.A., Polyakova, A.S., Fedorov, Y.V. et al. Fluorescent chemosensor for mercury(II) cations in an aqueous solution based on 4-acetylamino-1, 8-naphthalimide derivative containing the N-phenylazadithia-15-crown-5-ether receptor. Russ Chem Bull 70, 1939–1945 (2021). https://doi.org/10.1007/s11172-021-3300-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3300-6