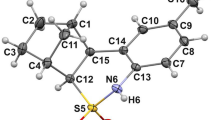
Abstract
Crystallization of three 4-arylsulfonyl-2(5H)-furanones from chloroform leads to the formation of a conglomerate of sulfone with para-tolyl substituent and racemic crystals of chloro and bromo analogs. The high degree of similarity of the crystal packings of a homochiral crystal and racemic compounds, viz., the similar type of the homochiral hydrogen-bonded chains and analogous three-dimensional homochiral layers additionally stabilized by the interactions of the type C=O…C=O and C-H…O, allowed us to suggest the presence of the second, “missing” form for each sulfone. A directed search for the “missing” forms revealed the existence of the racemic modification of sulfone with the para-tolyl fragment formed during a very slow crystallization of the compound from benzene. No conglomerates of bromo and chloro analogs were found. Topological analysis of the electron density distribution performed by quantum chemical calculations using density functional theory (PBE1PBE, 6–31G(d,p)) showed the higher energy favorability of intermolecular interactions in the homochiral chains as compared to the hypothetical heterochiral associates.
Similar content being viewed by others
References
J. Jacques, A. Collet, S. H. Wilen, Enantiomers, Racemates and Resolutions, Krieger Publishing Company, Malabar, FL, 1994, p. 447.
L. Laiserowitz, Acta Crystallogr., Sect. B, 1976, 32, 775.
E. Pidcock, Chem. Commun., 2005, 27, 3457.
A. Collet, M. Brienne, J. Jacques, Bull. Soc. Chim. Fr., 1972, 127.
L. Perez-Garcia, D. B. Amabilano, Chem. Soc. Rev., 2007, 36, 941.
P. A. Levkin, Yu. A. Strelenko, K. A. Lyssenko, V. Schurig, R. G. Kostyanovsky, Tetrahedron Asymmetry, 2003, 14, 2059.
P. A. Levkin, K. A. Lyssenko, V. Schurig, R. G. Kostyanovsky, Mendeleev Commun., 2003, 13, 106.
A. N. Kravchenko, G. K. Kadorkina, A. S. Sigachev, E. Yu. Maksareva, K. A. Lyssenko, P. A. Belyakov, O. V. Lebedev, O. N. Kharybin, N. N. Makhova, R. G. Kostyanovsky, Mendeleev Commun., 2003, 13, 114.
R. G. Kostyanovsky, V. Schurig, O. Trapp, K. A. Lyssenko, B. B. Averkiev, G. K. Kadorkina, A. V. Prosyanik, V. R. Kostyanovsky, Mendeleev Commun., 2002, 12, 137.
R. G. Kostyanovsky, I. A. Bronzova, K. A. Lyssenko, Mendeleev Commun., 2002, 12, 4.
R. G. Kostyanovsky, A. P. Avdeenko, S. A. Konovalova, G. K. Kadorkina, A. V. Prosyanik, Mendeleev Commun., 2000, 10, 16.
A. A. Bredikhin, Z. A. Bredikhina, D. V. Zakharychev, L. V. Konoshenko, Tetrahedron Asymmetry, 2007, 18, 1964.
A. V. Bodrov, L. E. Nikitina, V. A. Startseva, O. A. Lodochnikova, R. Z. Musin, O. I. Gnezdilov, Zh. Obshch. Khim., 2013, 83, 88 [Russ. J. Gen. Chem. (Engl. Transl.), 2013, 83, 80].
O. A. Lodochnikova, V. A. Startseva, E. N. Klimovitskii, I. A. Litvinov, Tez. dokl. V Nats. kristallokhim. konf. [Abstr. V National Crystallochem. Conf.], Kazan, 2009, p. 97 (in Russian).
A. O. Borissova, M. Yu. Antipin, H. A. Karapetyan, A. M. Petrosyan, K. A. Lyssenko, Mendeleev Commun., 2010, 20, 260.
R. F. W. Bader, Atoms in Molecules: A Quantum Theory, Oxford University Press, New York, 1990.
E. Espinosa, E. Mollins, C. Lecomte, Chem. Phys. Lett., 1998, 285, 170.
C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek, P. A. Wood, Mercury CSD 3.0.1, J. Appl. Crystallogr., 2008, 41, 466.
A. Choudhary, D. Gandla, G. R. Krow, R. T. Raines, J. Am. Chem. Soc., 2009, 131, 7244.
C. E. Jakobsche, A. Choudhary, S. J. Miller, R. T. Raines, J. Am. Chem. Soc., 2010, 132, 6651.
A. Choudhary, C. G. Fry, R. T. Raines, ARKIVOC, 2010, 8, 251.
P. Auffinger, F. A. Hays, E. Westhof, P. Shing Ho, PNAS, 2004, 101, 16789.
C. P. Brock, W. B. Schweizer, J. D. Dunitd, J. Am. Chem. Soc., 1991, 113, 9811.
Q. He, S. Rohani, J. Zhu, H. Gomaa, Cryst. Growth Des., 2010, 10, 5136.
L. Z. Latypova, A. R. Kurbangalieva, O. A. Lodochnikova, E. A. Berdnikov, G. A. Chmutova, Tez. dokl. XI Molodezhn konf. po organ. khim. [Abstr. XI Youth Conf. Org. Chem.] (Ekaterinburg, 23–29 November 2008), Ekaterinburg, 2008, 135 (in Russian).
G. M. Sheldrick, SADABS, University of Göttingen, Germany, 2004.
G. M. Sheldrick, Acta Crystallogr., 2008, A64, 112.
L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837.
APEX (Version 2.1), SAINTPlus. Data Reduction and Correction Program. Version 7.31A, Bruker Advansed X-Ray Solutsions. BrukerXS Inc., Madison, Wisconsin, USA, 2006.
A. L. Spek, J. Appl. Crystal., 2003, A36, 7.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, Gaussian 03, Revision B.04, Gaussian, Inc., Pittsburgh (PA), 2003.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865.
T. A. Keith, AIM ALL (version 10.05.04). URL: http://aim.tkgristmill.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor E. N. Klimovitskii.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 1218–1226, May, 2013.
Rights and permissions
About this article
Cite this article
Lodochnikova, O.A., Voronina, Y.K., Latypova, L.Z. et al. Crystallization of racemic 4-arylsulfonyl-2(5H)-furanones: reproducibility of homochiral associates, conditions for the spontaneous resolution of enantiomers and the formation of racemic compounds, the role of intermolecular interactions. Russ Chem Bull 62, 1218–1226 (2013). https://doi.org/10.1007/s11172-013-0167-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-013-0167-1